Figure 1.
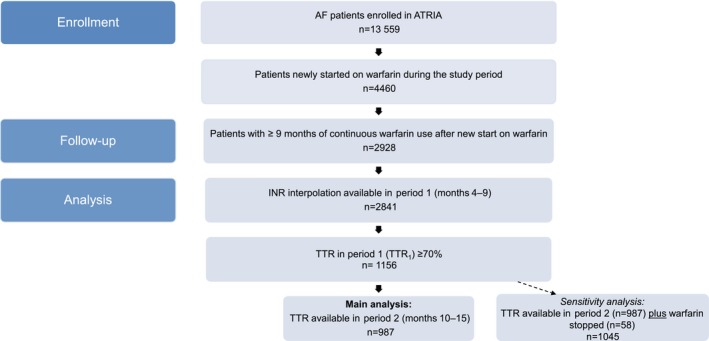
Selection of study cohort. Of the 13 559 patients in the ATRIA AF cohort, 4460 met the criteria for new starts on warfarin during the study period. Of these, 2928 had ≥9 months of continuous warfarin use, including our initial 3‐month dose‐finding period and our first period assessing TTR (months 4–9). There were 2841 patients who had interpolatable INR values (ie, calculable TTR values) in period 1. From these, we identified the 1156 patients newly started on warfarin and who had a TTR ≥70% in period 1 (TTR 1, months 4–9). The primary analysis focused on the 987 patients who had TTR 1 ≥70% and had a calculable TTR in period 2 (TTR 2, months 10–15). In a sensitivity analysis, we also included the 58 patients with TTR 1 ≥70% who discontinued warfarin in period 2. We did not include the following categories of patients in the analysis of TTR 2: (1) died in period 2 (n=16), (2) discontinued warfarin in period 2 but restarted warfarin within 1 year (n=42), (3) period 2 follow‐up was incomplete because the study ended (n=33), (4) no interpolatable INR values in period 2 (n=17), and (5) disenrolled in the health plan during period 2 (n=3). AF indicates atrial fibrillation; ATRIA, anticoagulation and risk factors in atrial fibrillation; INR, International Normalized Ratio; TTR, time in the therapeutic range.
