Abstract
Background
Whether the remote myocardium of reperfused ST‐segment elevation myocardial infarction (STEMI) patients plays a part in adverse left ventricular (LV) remodeling remains unclear. We aimed to use automated extracellular volume fraction (ECV) mapping to investigate whether changes in the ECV of the remote (ECVR emote) and infarcted myocardium (ECVI nfarct) impacted LV remodeling.
Methods and Results
Forty‐eight of 50 prospectively recruited reperfused STEMI patients completed a cardiovascular magnetic resonance at 4±2 days and 40 had a follow‐up scan at 5±2 months. Twenty healthy volunteers served as controls. Mean segmental values for native T1, T2, and ECV were obtained. Adverse LV remodeling was defined as ≥20% increase in LV end‐diastolic volume. ECVR emote was higher on the acute scan when compared to control (27.9±2.1% vs 26.4±2.1%; P=0.01). Eight patients developed adverse LV remodeling and had higher ECVR emote acutely (29.5±1.4% vs 27.4±2.0%; P=0.01) and remained higher at follow‐up (28.6±1.5% vs 26.6±2.1%; P=0.02) compared to those without. Patients with a higher ECVR emote and a lower myocardial salvage index (MSI) acutely were significantly associated with adverse LV remodeling, independent of T1Remote, T1Core and microvascular obstruction, whereas a higher ECVI nfarct was significantly associated with worse wall motion recovery.
Conclusions
ECVR emote was increased acutely in reperfused STEMI patients. Those with adverse LV remodeling had higher ECVR emote acutely, and this remained higher at follow‐up than those without adverse LV remodeling. A higher ECVR emote and a lower MSI acutely were significantly associated with adverse LV remodeling whereas segments with higher ECVI nfarct were less likely to recover wall motion.
Keywords: extracellular volume fraction, left ventricular remodeling, ST‐segment elevation myocardial infarction, T1 mapping, T2 mapping
Subject Categories: Magnetic Resonance Imaging (MRI)
Introduction
Although mortality after an acute ST‐elevation myocardial infarction (STEMI) is in decline, the onset of post–myocardial infarct (MI) heart failure is still significant.1, 2, 3 MI size,4, 5 presence of microvascular obstruction (MVO),6, 7 and myocardial salvage8, 9 assessed by cardiovascular magnetic resonance (CMR) performed in the first few days after reperfusion in STEMI patients have all been shown to be strong predictors of adverse left ventricular (LV) remodeling and development of heart failure.
Whether changes in the extracellular matrix (ECM) in the noninfarcted remote myocardium in STEMI patients reperfused by primary percutaneous coronary intervention (PPCI) is linked to adverse LV remodeling remains incompletely understood.10, 11, 12, 13, 14 Although native T1 mapping14 and postcontrast T1 mapping13 CMR have been used to interrogate remote myocardial ECM, the availability of automated extracellular volume fraction (ECV) mapping CMR provides a more robust method for quantifying not only focal fibrosis, but also diffuse interstitial expansion in the myocardium15, 16 given that native and postcontrast T1 maps are coregistered and motion corrected, thereby improving the quality of the generated automated maps.17
Using the latest coregistered and motion‐corrected T1 mapping technology to create accurate automated ECV maps, we set out to investigate, first, whether changes in ECV in the remote myocardium occurred in the current era and whether they were associated with adverse LV remodeling and, second, whether ECV of the infarct zone was a better predictor of wall motion recovery than transmural extent of the infarct after reperfused STEMI.
Methods
Study Population
This was a prospective, single‐center study of acute STEMI patients reperfused by PPCI. Fifty patients were recruited over a 12‐month period, and 20 age‐ and sex‐matched healthy volunteers served as control (all free of cardiovascular disease). All participants were scanned at the same center and on the same scanner. Diagnosis and treatment of STEMI were as per current guidelines.18, 19 Study exclusion criteria were previous MI and standard recognized contraindications to CMR (eg, ferromagnetic implants, claustrophobia, and estimated glomerular filtration rate <30 mL/min). All patients provided informed written consent. The UK National Research Ethics Service approved this study.
Imaging Acquisition
All CMR scans were performed on a 1.5 Tesla scanner (MAGNETOM Avanto; Siemens Medical Solutions, Malvern, PA) using a 32‐channel phased‐array cardiac coil.
Imaging protocol
In the STEMI cohort, all patients had T2 maps acquired from base to apex during the acute scan. As for native T1 and postcontrast T1 maps, 30 patients had whole LV short‐axis coverage and the remaining 10 had 3 (basal, mid, and apical) LV short‐axis T1 maps acquired during both scans. The 20 healthy volunteers had 1 midventricular short‐axis native T1, T2, and postcontrast T1 maps acquired. All participants had whole heart coverage LV short‐axis cines and late gadolinium enhancement (LGE) acquired.
Native T1 mapping
T1 maps (Work In Progress 448B; Siemens Healthcare, Erlangen, Germany) were acquired using a steady state free precession (SSFP)‐based modified look‐locker inversion recovery (MOLLI) sequence. A 5s(3s)3s modified MOLLI sampling protocol was used to ensure more‐complete recovery of the inversion pulse at higher heart rates by acquiring a set of images for at least 5 seconds after the first inversion pulse, followed by a 3‐second pause and then acquiring a set of images after the second inversion pulse for at least 3 seconds.20 The acquisition parameters were: pixel bandwidth, 977 Hz/pixel; echo time=1.1 ms; flip angle=35 degrees; matrix=256×144; and slice thickness=6 mm. Motion correction and a nonlinear least‐square curve fitting were performed with the set of images acquired at different inversion times to generate a pixel‐wise colored T1 map by the scanner.
T2 mapping
T2 maps (Work In Progress 448B; Siemens Healthcare, Erlangen, Germany) were acquired as previously described.21 In brief, 3 single‐shot images at different T2 preparation times (0, 24, and 55 ms, respectively) using the following parameters were acquired: pixel bandwidth, 930 Hz/pixel; echo time=1.1 ms; repetition time=3× R‐R interval; flip angle=70 degrees; acquisition matrix=116×192; and slice thickness=6 mm. A colored T2 map consisting of pixel‐wise T2 values was generated after fitting to estimate T2 relaxation times and motion correction by the scanner.
Late gadolinium enhancement
LGE imaging was acquired with a standard segmented “fast low‐angle shot” 2‐dimensional inversion‐recovery gradient echo sequence or a respiratory motion‐corrected, free‐breathing, single‐shot, SSFP‐averaged, phase‐sensitive inversion recovery sequence22, 23 at 10 to 15 minutes after the injection of 0.1 mmol/kg of gadoterate meglumine (Gd‐DOTA marketed as Dotarem; Guerbet S.A., Paris, France).
Postcontrast T1 mapping
Postcontrast T1 maps (Work In Progress 448B; Siemens Healthcare, Erlangen, Germany) were obtained using the 4s(1s)3s(1s)2s sampling protocol (to improve the accuracy of T1s in the 200‐ to 600‐ms range as previously described20) 15 minutes after contrast injection (0.1 mmol/kg of Dotarem) using similar acquisition parameters as for native T1 maps.
ECV maps
The previously described and validated automated method for producing a pixel‐wise ECV map was used.17 In brief, this method corrects for respiratory motion attributed to poor breath holding as well as patient movement between breath holds and relies on coregistration of the native and postcontrast T1 pixel maps. Each patient had hematocrit checked at the time of the scan and the ECV was estimated using the following formula24:
An offline software (ECV Mapping Tool, Version 1.1) subsequently generated pixel‐wise ECV maps using a variety of postprocessing steps as recently described.17
Imaging Analysis
All imaging analysis was performed using CVI42 software (version 5.1.2[303]; Circle Cardiovascular Imaging, Calgary, Alberta, Canada).
Wall motion analysis
Segmental wall motion on the short‐axis cine images were visually scored by 2 experienced investigators (H.B., S.R.) as “0” for normal; “1” for mild/moderate hypokinesis; “2” for severe hypokinesis; “3” for akinesis; and “4” dyskinesis25 and displayed as per the 16‐segment American Heart Association (AHA) classification.26 Wall motion recovery was defined as an improvement in wall motion score by 1. Segmental wall end‐diastolic and end‐systolic wall thickness, wall thickening, and wall motion were derived from averaging the mean thicknesses from 100 chords for each short‐axis slice, covering the whole LV, and also displayed as per the modified 16‐segment AHA model.
MI and area‐at‐risk quantification
MI size was quantified using a threshold of 5 SDs27 above the mean remote myocardium and expressed as a percentage of the LV. The area‐at‐risk (AAR) was assessed from the T2 maps using a threshold of 2 SDs above the mean remote myocardium and expressed a percentage of the whole LV.28, 29 Areas of hypointense core of MVO were included as part of the MI zone, and AAR and MVO from the LGE images were quantified and expressed in grams. Transmural extent of LGE was expressed by averaging the values from 100 chords from each short‐axis slice to obtain the mean transmural extent of LGE for each of the 16 AHA segments. Figure 1 shows an example of a mid‐LV short‐axis ECV map of an acute anterior STEMI with MVO and the corresponding follow‐up ECV map.
Figure 1.
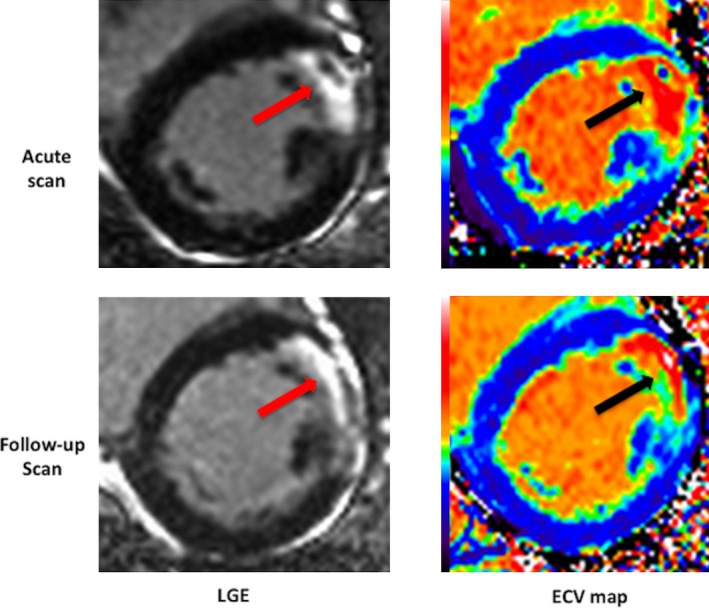
Example of LGE images and ECV maps of an acute and follow‐up scan of a patient with an anterior STEMI. The arrows show the area of LGE with a core of MVO on the acute scan and the corresponding chronic LGE and ECV. ECV indicates extracellular volume fraction; LGE, late gadolinium enhancement; MVO, microvascular obstruction; STEMI, ST‐segment elevation myocardial infarction.
Analysis of the T1, T2, and ECV maps
Endocardial and epicardial borders were manually delineated on the native T1 maps and T2 maps and copied on the ECV maps. The left ventricular outflow track and apical LV short‐axis slices were excluded, and a 10% erosion of the wall thickness was applied to the endocardial and epicardial borders to minimize partial volume effects. Mean segmental T1, T2, and ECV values were then generated and displayed as bull's‐eye plots using the 16 AHA segments (Figure 2). For assessing intermethod agreement, manual regions of interest (ROIs) were also drawn with care to avoid partial volume effects in the remote myocardium and infarcted segments of 10 patients for comparison with mean segmental values for ECV. The remote myocardium was defined as the AHA segment 180 degrees from the infarct territory with normal wall motion and no LGE. T1, T2, and ECV values in the remote myocardium were represented by T1Remote, T2Remote, and ECVRemote. T1 values of the core (T1Core) of the infarct was obtained by manually drawing the ROI within the hypoenhanced regions inside areas of hyperenhancement within 2 SDs of the remote myocardium on the T1 maps and are represented as T1Core. Mean segmental ECV of the infarcted segments is represented as ECVInfarct. Adverse LV remodeling was defined as a ≥20% increase in end‐diastolic volume (EDV) between the acute and follow‐up scans.14
Figure 2.
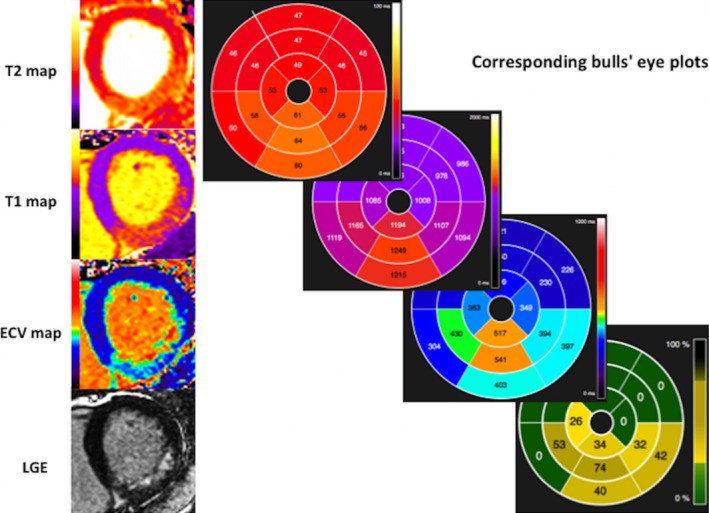
Example of generated bull's eye plots with AHA segments from the maps and LGE images. AHA indicates American Heart Association; ECV, extracellular volume fraction; LGE, late gadolinium enhancement.
Statistical Analysis
Statistical analysis was performed using SPSS software (version 22; IBM Corp, Chicago, IL). Test for normality was performed using the Shapiro–Wilk Test. Continuous data are expressed as mean±SD or median (interquartile range) and compared with the paired Student t test/Wilcoxon signed‐rank test or unpaired Student t test/Mann–Whitney U test, where appropriate. Categorical data are reported as frequencies and percentages. Interobserver and intraobserver variability for ECVRemote and ECVInfarct was assessed in 10 patients and expressed as intraclass correlation coefficient (ICC) and 95% CI and bias ±2 SDs (for limits of agreement) using Bland–Altman analysis. Receiver operating characteristic (ROC) analyses were performed to assess the diagnostic performance for LGE, T1, T2, and ECV on the acute scan for predicting segmental wall motion recovery for the AHA segments with LGE, and this was repeated for segments with LGE, but without MVO. Univariable and multivariable linear regression analyses were also performed to identify associates of adverse remodeling. To take into consideration potential within‐subject interaction of some segments with the independent variables, R software (version 3.2.3; R Foundation for Statistical Computing, Vienna, Austria; available at: http://www.R-project.org/) was used for clustered ROC analysis by the method described by Obuchowski30 to assess the performance of T1, T2, ECV, and LGE to predict wall motion recovery, and a linear mixed‐effects model was used to identify predictors of segmental wall motion recovery after adjusting interaction among segments within the same patients. All statistical tests were 2‐tailed, and P<0.05 was considered statistically significant.
Results
Figure 3 illustrates the STEMI patients’ screening and recruitment process. Of 50 STEMI patients recruited into the study, 48 completed the first CMR at 4±2 days post‐PPCI and 40 had a follow‐up scan at 5±2 months (2 patients did not complete the scan because of unexpected claustrophobia, 8 were lost to follow‐up). There was no difference in the main acute CMR characteristics apart from a higher EDV between the 40 patients who had paired acute and follow‐up scans and those who only had the acute scan, as shown in Table 1. Mean age of STEMI patients was 59±13 years, and 88% were male. Further details regarding patient characteristics are listed in Table 2. Mean age of healthy volunteers was 60±10 years, and 90% were male. A total of 10% of the T1, T2, and ECV maps had to be excluded from the analysis because of artifacts, partial volume effects, and/or being nondiagnostic, as previously described.17
Figure 3.
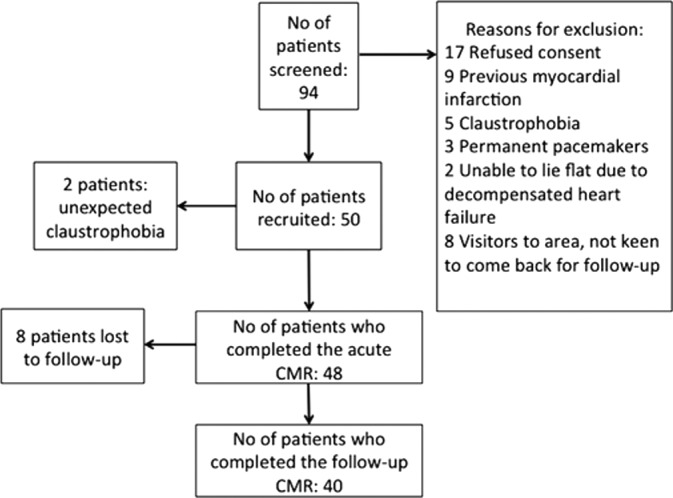
STEMI patients screening and recruitment flow chart. CMR indicates cardiovascular magnetic resonance imaging; STEMI, ST‐segment elevation myocardial infarction.
Table 1.
Acute CMR Characteristics Between Patients Who Completed Paired Acute and Follow‐up Scans (n=40) and Patients Who Only Had the Acute Scan (n=8)
| Patients With Paired CMR (n=40) | Patients With Acute CMR Only (n=8) | P Value | |
|---|---|---|---|
| Acute LV EDV, mL | 172±38 | 135±28 | 0.01a |
| Acute indexed LV EDV, mL/m2 | 87±13 | 73±13 | 0.01a |
| Acute LV ESV, mL | 90±30 | 66±20 | 0.04a |
| Acute indexed LV ESV, mL/m2 | 45±13 | 36±11 | 0.07 |
| Acute LV EF, % | 49±8 | 52±8 | 0.32 |
| Acute LV mass, g | 111 (92–124) | 137 (86–154) | 0.28 |
| Acute MI size, % of LV | 28 (14.8–38.0) | 24.0 (23.0–25.8) | 0.52 |
| AAR, % of LV | 42.0±12.0 | 46.4±10.9 | 0.34 |
| MVO, % | 26 (65%) | 5 (63%) | 0.60 |
| Acute T2Remote, ms | 50 (48–52) | 49 (48–54) | 0.78 |
| Acute T1Remote, ms | 1026 (990–1077) | 1043 (963–1095) | 0.80 |
| Acute ECVRemote, % | 28.1 (26.2–29.7) | 27.6 (26.2–31.2) | 0.73 |
AAR indicates area‐at‐risk; CMR, cardiovascular magnetic resonance imaging; EDV, end diastolic volume; EF, ejection fraction; ESV, end systolic volume; LV, left ventricle; MVO, microvascular obstruction.
Denotes statistical significance at P<0.05.
Table 2.
Clinical Characteristics of STEMI Patients (n=40)
| Details | No. |
|---|---|
| No. of patients | 40 |
| Male (%) | 35 (88) |
| Age, y | 59±13 |
| Diabetes mellitus (%) | 8 (20) |
| Hypertension (%) | 14 (35) |
| Smoking (%) | 12 (30) |
| Dyslipidemia (%) | 14 (35) |
| Chest pain onset to PPCI time, min | 267 [122–330] |
| Infarct artery (%) | |
| LAD | 24 (60) |
| RCA | 14 (35) |
| Cx | 2 (5) |
| Pre‐PPCI TIMI flow (%) | |
| 0 | 33 (83) |
| 1 | 0 (0) |
| 2 | 3 (8) |
| 3 | 4 (10) |
| Post‐PPCI TIMI flow (%) | |
| 0 | 1 (3) |
| 1 | 0 (0) |
| 2 | 8 (20) |
| 3 | 31 (77) |
| Treatment—during PPCI (%) | |
| Aspirin | 100 (100) |
| Clopidogrel | 24 (60) |
| Ticagrelor | 16 (40) |
| Heparin | 36 (90) |
| Bivalirudin | 11 (28) |
| Glycoprotein IIbIIIa inhibitors | 11 (28) |
| Treatment—on discharge (%) | |
| Dual antiplatelet therapy | 40 (100) |
| Beta‐blockers | 40 (100) |
| ACEI/ARB | 40 (100) |
| Statin | 39 (98) |
| MRA | 10 (25) |
ACEI/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; Cx, circumflex artery; LAD, left anterior descending artery; MRA, mineralocorticoid receptor antagonist; PPCI, primary percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST‐segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
As expected, LV EDV and end‐systolic volume (ESV) were significantly higher for STEMI patients (on both the acute and follow‐up scans) when compared to the controls (Table 3), although there was no significant difference in LV mass. Mean MI size in STEMI patients was 27.4±14.6% of the LV, and mean AAR was 42.0±12.0% of the LV, giving a mean myocardial salvage index (MSI) of 0.37±0.27 of the LV. As expected, there was a significant regression in MI size between the acute scan and the follow‐up scan (27.4±14.6% to 19.5±10.5%; P<0.001). Twenty‐six of 40 (65%) of the included patients had MVO.
Table 3.
CMR Findings (n=40 STEMI Patients and n=20 Controls)
| Controls (n=20) | Acute Scan (n=40) | Follow‐up Scan (n=40) | Change Between Acute and Follow‐up | P Value | |
|---|---|---|---|---|---|
| LV EDV, mL | 148±34 | 172±38 | 182±49 | −9±25 |
0.02a
0.01b |
| LV ESV, mL | 55±16 | 90±30 | 88±38 | 2±24 |
0.001a
0.001b |
| LV EF, % | 63±5 | 49±8 | 53±10 | −5±8 |
0.001a
0.001b |
| LV mass, g | 108±21 | 112±35 | 104±26 | 8±27 | NS |
| LV wall thickness in remote myocardium—diastole, mm | 7.2±0.7 | 7.1±1.3 | 6.7±1.3 | 0.4±1.3 | NS |
| LV wall thickness in remote myocardium—systole, mm | 12.0±1.3 | 12.0±1.8 | 11.2±1.8 | 0.8±2.3 | NS |
| LV wall thickening in remote myocardium, % | 66±14 | 77±40 | 75±26 | 2±43 | NS |
| LV wall motion in remote myocardium, mm | 7.7±2.5 | 9.2±2.6 | 8.9±2.6 | 0.3±2.9 |
0.04a
0.10b |
| Infarct size, % of LV | NA | 27.4±14.6 | 19.5±10.5 | 7.9±7.2 | 0.0001 |
| Infarct size, g | NA | 20.2±13.6 | 14.4±9.4 | 5.8±5.9 | 0.0001 |
| AAR, % of LV | NA | 42.0±12.0 | NA | NA | |
| T2Remote, ms | 50±4 | 50±3 | 48±2 | 1±3 |
0.94a
0.001b |
| T2Infarct, ms | NA | 65±5 | 57±5 | 9±7 | 0.0001 |
| T2Core, ms | NA | 51±5 | 47±3 | 4±5 | 0.001 |
| T1Remote, ms | 1000±25 | 1032±51 | 1004±39 | 29±52 |
0.001a
0.66b |
| T1Infarct, ms | NA | 1245±75 | 1141±53 | 104±88 | 0.0001 |
| T1Core, ms | NA | 1025±89 | 1029±52 | −5±79 | 0.74 |
| ECVRemote, % | |||||
| Whole cohort (n=40) | 26.4±2.1 | 27.9±2.1 | 27.0±2.1 | 0.9±1.9 |
0.01a
0.30b |
| With adverse LV remodeling (n=8) | NA | 29.5±1.4 | 28.6±1.5 | 0.9±2.2 | 0.27 |
| Without adverse LV remodeling (n=32) | NA | 27.4±2.0 | 26.6±2.1 | 0.9±1.9 | 0.02 |
| ECVInfarct, % | NA | 69.2±9.6 | 70.4±19.9 | −1.2±18.3 | 0.71 |
AAR indicates area‐at‐risk; CMR, cardiovascular magnetic resonance imaging; ECV, extracellular volume fraction; EDV, end diastolic volume; EF, ejection fraction; ESV, end systolic volume; LV, left ventricle; MVO, microvascular obstruction; NA, not applicable; NS, not statistically significant; STEMI, ST‐segment elevation myocardial infarction.
Control vs acute scan.
Control vs follow‐up scan.
Intraobserver and Interobserver Variability for ECV Measurements
There was less intraobserver and interobserver variability when ECVRemote and ECVInfarct were assessed by mean segmental analysis when compared to manual ROI (Table 4). For intraobserver measurements, the limits of agreement for ECVRemote were ±1.22% when using mean segmental values compared to ±1.59% when using manual ROI. For interobserver measurements, the limits of agreement for ECVRemote were ±0.99% when using mean segmental values compared to ±1.40% when using manual ROI. The limits of agreement for the two techniques were even wider for ECVInfarct (Table 4).
Table 4.
Intraobserver and Interobserver Variability for ECV Using 2 Different Techniques (Mean Segmental Values and Manual ROI; n=10)
| Intraclass Correlation Coefficient (95% CI) | Bias±Limits of Agreement (%) | |
|---|---|---|
| Intraobserver variability (n=10) | ||
| ECVRemote | ||
| Mean segmental values | 0.994 (0.976–0.998) | 0.11±1.22 |
| Manual ROI | 0.981 (0.922–0.995) | 0.42±1.59 |
| ECVInfarct | ||
| Mean segmental values | 0.992 (0.967–0.998) | 0.53±2.44 |
| Manual ROI | 0.972 (0.886–0.993) | 0.13±5.02 |
| Interobserver variability (n=10) | ||
| ECVRemote | ||
| Mean segmental values | 0.996 (0.984–0.999) | 0.10±0.99 |
| Manual ROI | 0.989 (0.958–0.997) | 0.18±1.40 |
| ECVInfarct | ||
| Mean segmental values | 0.991 (0.949–0.998) | 0.81±2.21 |
| Manual ROI | 0.963 (0.850–0.991) | 0.10±5.96 |
ECV indicates extracellular volume fraction; ROI, region of interest.
Changes in the Remote Myocardium
For both the acute and follow‐up scans, there were no differences in LV diastolic and systolic wall thickness and LV wall thickening in the remote myocardium between STEMI patients and controls (Table 3). However, on the acute scan, LV wall motion in the remote myocardium was higher in STEMI patients when compared to controls (9.2±2.6 vs 7.7±2.5 mm; P=0.04), but there was no statistically significant difference between them on the follow‐up scan (8.9±2.6 vs 7.7±2.5 mm; P=0.10; Table 4).
There were no significant differences in the T2Remote values between STEMI patients and controls on either the acute scan or the follow‐up scans. Native T1Remote and ECVRemote were both significantly higher in STEMI patients when compared to controls on the acute scan (T1: 1032±51 vs 1000±25 ms; P=0.001; ECV: 27.9±2.1% vs 26.4±2.1%; P=0.01), but this difference was not present on the follow‐up scan (T1: 1004±39 vs 1000±25 ms; P=0.66; ECV: 27.0±2.1% vs 26.4±2.1%; P=0.30; Table 3).
LV Remodeling
Of 40 STEMI patients who had a follow‐up scan, 8 (20%) patients had adverse LV remodeling. In these 8 patients, the ECVRemote was higher acutely when compared to those 32 without adverse LV remodeling (29.5±1.4% vs 27.4±2.0%; P=0.01), and this difference in ECVremote persisted on the follow‐up scan (28.6±1.5% vs 26.6±2.1%; P=0.02). There were no significant differences in T2Remote between those with and without adverse LV remodeling both on the acute and follow‐up scans (Figure 4).
Figure 4.
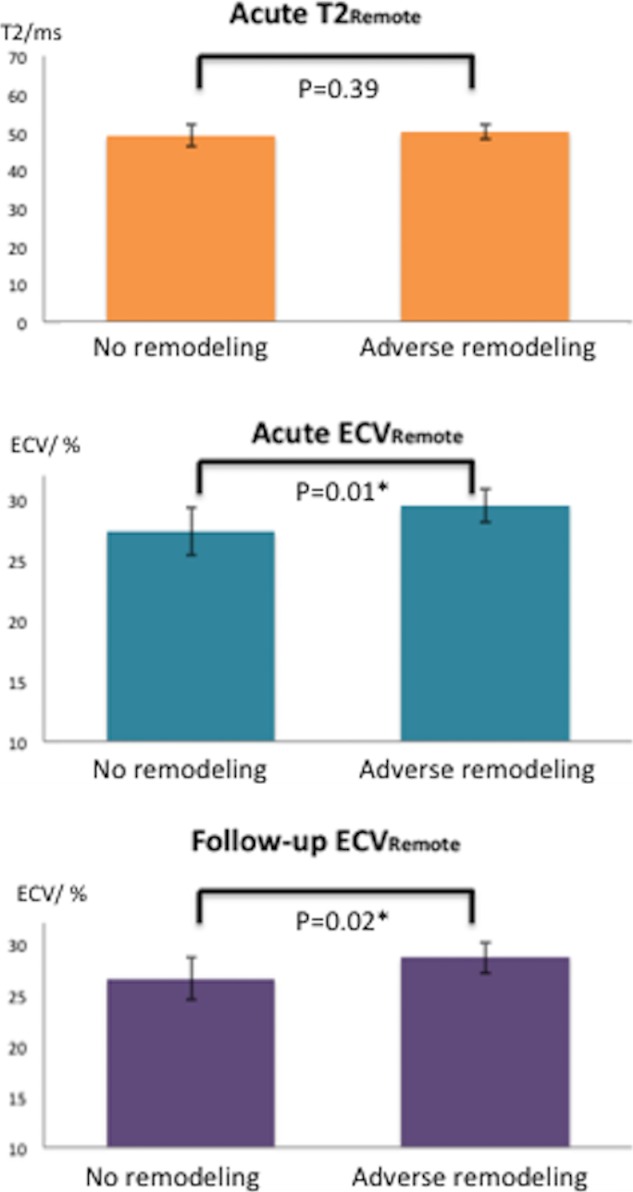
T2 and ECV of the remote myocardium in STEMI patients with (n=8) and without LV remodeling (n=32). ECV indicates extracellular volume fraction; LV, left ventricular; STEMI, ST‐segment elevation myocardial infarction. *Denotes statistical significance at P<0.05.
Multiparametric CMR Prediction of Remodeling
A percentage increase in LV EDV as a continuous variable was used as a surrogate for adverse LV remodeling for univariable and multivariable linear regression analysis. MI size quantified by LGE (R 2=0.36; coefficient, 0.64; 95% CI, 0.37–0.9; P=0.0001) was the single most significant predictor of adverse LV remodeling after adjusting for remote myocardial T1, MVO, and LV EDV on the acute scan in a multivariable analysis. In order to account for both MI size and AAR, MSI was used in the regression model instead of MI size. T1Remote and ECVRemote, T1Core, MSI, and MVO on the acute scan were significantly associated with adverse LV remodeling on univariable analysis, and these were then included in a multivariable analysis. MSI and then ECVRemote were most associated with adverse LV remodeling after adjusting for T1Remote, T1Core and MVO on the acute scan (Table 5).
Table 5.
Univariable and Multivariable Associates of LV Remodeling (n=40)
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Regression Slope | R 2 | P Value | Regression Slope | P Value | |
| MSI | −29 | 0.25 | 0.001 | −25 | 0.008 |
| ECV remote | 3.5 | 0.24 | 0.001 | 2.6 | 0.03 |
| MVO, g | 2.7 | 0.26 | 0.001 | NS | |
| T1 remote | 0.13 | 0.20 | 0.03 | NS | |
| T1Core | −0.06 | 0.14 | 0.001 | NS | |
ECV indicates extracellular volume fraction; LV, left ventricle; MSI, myocardial salvage index; MVO, microvascular obstruction; NS, not statistically significant.
ECV and Segmental Recovery of LV Systolic Function
Two hundred sixty‐three of 640 segments had LGE and abnormal wall motion. The mean ECVInfarct of all segments with LGE on the acute scan was 50.5±9.4%. MVO was present in 77 of these 263 segments, and it was negatively associated with segmental LV wall motion recovery after adjusting for transmural extent of LGE and for the interaction between segments with MVO and patients (77 segments; only 24% recovered; P<0.001; R 2=0.80). Because gadolinium contrast failed to reach pseudoequilibrium in areas of MVO, analysis for segmental LV wall motion recovery was performed with and without segments containing MVO. ECVInfarct was performed as well as transmural extent of LGE on clustered ROC curve analysis (area under the curve [AUC], 0.68; 95% CI, 0.59–0.77 vs 0.72; 95% CI, 0.64–0.80; P=0.23 for comparison of both ROC curves), whereas mean segmental T1 and T2 were not good tests to predict improvement in segmental LV wall motion (T1: AUC, 0.45; 95% CI, 0.37–0.54; P=0.60; T2: AUC, 0.54; 95% CI, 0.44–0.64; P=0.15). After excluding segments with MVO (77 segments), this relationship was maintained between ECVInfarct and transmural extent of LGE (AUC, 0.63; 95% CI, 0.52–0.73 vs 0.63; 95% CI, 0.53–0.73; P=0.89 for ROC curve comparison). A lower ECVInfarct was found to be a significant predictor of wall motion recovery after adjusting for transmural extent of LGE and interaction among segments within the same patients (P=0.039; R 2=0.48).
Discussion
The major findings of this study were as follows: (1) We showed that ECVRemote in STEMI patients is acutely elevated, and this elevation persisted in those STEMI patients that developed adverse remodeling at 5 months; (2) MSI and ECVRemote in the remote myocardium were most associated with adverse LV remodeling after adjusting for T1Remote, T1Core, and MVO on the acute scan; and (3) ECVInfarct was found to be a significant predictor of LV wall motion recovery after adjusting for transmural extent of LGE.
There was no statistical difference in T2 values that were observed in the remote myocardium of STEMI patients when compared to controls, suggesting that the increased ECV was probably not attributed to myocardial edema, although there was a trend toward the T2Remote values of STEMI patients being higher. There is a possibility that it may have been attributed to an expansion of the intravascular compartment from increased myocardial blood flow associated with the compensatory increase in LV wall motion observed in the remote myocardium.
Of note, this increase in ECVRemote only persisted in those STEMI patients who went on to develop adverse LV remodeling. In a murine model of acute MI, Tsuda et al.11 found molecular and immune‐histochemical evidence of interstitial fibrosis in the remote myocardium as early as 72 hours post‐MI. Volders et al.10 provided postmortem histological evidence of an increase in interstitial collagen in the remote myocardium of infarcted patients when compared to control. It may be possible that the increase in ECVRemote in this subset of patients represent early interstitial fibrosis. However, Marijianowski et al.12 showed that post‐MI LV remodeling in patients with end‐stage heart failure undergoing transplant was not associated with interstitial fibrosis in the remote myocardium.
Chan et al.13 used postcontrast T1 in 25 acute STEMI patients and found evidence of early remote systolic dysfunction and expansion of the ECM, which persisted in the chronic stage, and their findings partly differ from ours. We did not find evidence of systolic dysfunction in the remote myocardium, but, paradoxically, found an increase in wall motion in the remote myocardium in the acute scan only. These differences may partly be attributed to the smaller number of patients and methodology used in their study (definition of a remote sector: any segment without LGE as defined in their study may potentially include adjacent segments with edema and stunning; they only analyzed base, mid, and an apical cine compared to whole‐LV short‐axis analysis and using averaged values of the midventricular segments in our study). Furthermore, not all STEMI patients showed persistence of ECVRemote expansion at follow‐up when using automated ECV maps and may be attributed to the fact that we included more patients with a wider range of MI size as a percentage of the LV (acute MI size in our study 27.4±14.6% vs 19.2±10.5% in their study) and the higher use of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB) and mineralocorticoid receptor antagonist (MRA) in our study (ACEI/ARB, 92% vs 100%: MRA, 16% vs 25%).
Carrick et al.14 demonstrated that increased native T1 values in remote myocardium of reperfused STEMI patients were independently associated with adverse LV remodeling after adjusting for LV EDV, MI size, and MVO on the acute scan. In a separate publication, Carrick et al.31 also showed that native T1 of the hypointense infarct core was inversely associated with the risk of all‐cause mortality or hospitalization for heart failure in the same cohort of patients, although they did not adjust for native T1 of the remote myocardium. In contrast, in our cohort, we showed that MI size was the single most significant predictor of adverse LV remodeling after adjusting for similar parameters, including native T1 of the remote myocardium. Although MI size is known to be a strong predictor of outcome,4, 5 MSI is a more‐sensitive marker to assess the effectiveness of a reperfusion strategy32, 33 in randomized, controlled trials and it was recently shown that MSI significantly reduced sample size.34 Therefore, we opted to use MSI instead of MI size expressed as a percentage of the LV. We found that acute ECVRemote together with MSI were also strongly associated with LV remodeling after adjusting for both T1Remote and T1Core and MVO. There was no evidence of edema of the remote myocardium by T2 mapping, even in those patients who subsequently developed LV remodeling.
Although the sample size was smaller in our study, our findings may also differ from the studies by Carrick et al.,14, 31 given that we used mean segmental values for T1, T2, and ECV rather than manual ROI (the ICC for reliability of remote T1 in Carrick et al.14 was 0.92 and had quite a wide 95% CI [0.80, 0.97]). Furthermore, the majority of our patients (>70%) had whole LV coverage for the T1 maps, and ECV maps and were averaged to derive the AHA segmental values and therefore were more likely to be a better representation of the changes in the remote myocardium.
For myocardial segments with LGE, ECVInfarct was as good a predictor as transmural LGE for LV wall motion recovery. For segments without MVO, ECVInfarct was a significant predictor of segmental LV systolic recovery after adjusting for LGE. Given the heterogeneity of LGE, we chose to use mean segmental ECV values to minimize sampling errors and partial volume effects, and this approach may be a better reflection of the ECV matching the corresponding transmural extent of LGE per segment on both the acute and follow‐up scan. The availability of ECV maps could provide further quantification of the infarct severity and complement transmural extent of LGE to predict wall motion recovery.
Limitations
To our knowledge, this is the first study to use automated ECV maps in this setting, which is more accurate to assess changes in myocardial extracellular volume17 and also the first study to investigate the changes occurring in the remote and infarct‐related segments by ECV in paired CMR scans of STEMI patients performed acutely and after a follow‐up of 5 months. However, our study is not without limitations. We only included a relatively small number of patients, and therefore we were unable to determine the impact of changes in extracellular volume on major adverse cardiovascular events. We did not collect biochemical markers for inflammation, remodeling, and fibrosis to correlate with CMR findings.
Summary and Conclusions
ECVRemote was increased acutely in STEMI patients reperfused by PPCI when compared to healthy controls. For those patients who developed adverse LV remodeling, ECVRemote acutely was higher than those who did not develop adverse LV remodeling and remained persistently higher at follow‐up. Patients with higher ECVRemote and lower MSI acutely were more likely to develop adverse LV remodeling, whereas segments with higher ECVInfarct were less likely to have wall motion recovery.
Sources of Funding
This work was supported by the British Heart Foundation (FS/10/039/28270), the Rosetrees Trust, and the National Institute for Health Research University College London Hospitals Biomedical Research Center
Disclosures
None.
Acknowledgments
We express our gratitude to the staff and patients at the UCLH Heart Hospital and Peter Weale for providing us with the Work In Progress investigational sequences under a research collaboration agreement with Siemens Healthcare.
(J Am Heart Assoc. 2016;5:e003555 doi: 10.1161/JAHA.116.003555)
References
- 1. Torabi A, Cleland JG, Khan NK, Loh PH, Clark AL, Alamgir F, Caplin JL, Rigby AS, Goode K. The timing of development and subsequent clinical course of heart failure after a myocardial infarction. Eur Heart J. 2008;29:859–870. [DOI] [PubMed] [Google Scholar]
- 2. Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in‐hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. [DOI] [PubMed] [Google Scholar]
- 3. Levi F, Lucchini F, Negri E, La Vecchia C. Trends in mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world. Heart. 2002;88:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli‐Ducci C, Kansal P, Carr JC, Holly TA, Lloyd‐Jones D, Klocke FJ, Bonow RO. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end‐systolic volume index: prospective cohort study. Heart. 2008;94:730–736. [DOI] [PubMed] [Google Scholar]
- 5. Roes SD, Kelle S, Kaandorp TA, Kokocinski T, Poldermans D, Lamb HJ, Boersma E, van der Wall EE, Fleck E, de Roos A, Nagel E, Bax JJ. Comparison of myocardial infarct size assessed with contrast‐enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol. 2007;100:930–936. [DOI] [PubMed] [Google Scholar]
- 6. Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta‐analysis. JACC Cardiovasc Imaging. 2014;7:940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, Cottin Y, Atar D, Buser P, Wu E, Lee D, Bodi V, Klug G, Metzler B, Delewi R, Bernhardt P, Rottbauer W, Boersma E, Zijlstra F, van Geuns RJ. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014;7:930–939. [DOI] [PubMed] [Google Scholar]
- 8. Eitel I, Desch S, Fuernau G, Hildebrand L, Gutberlet M, Schuler G, Thiele H. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:2470–2479. [DOI] [PubMed] [Google Scholar]
- 9. Masci PG, Ganame J, Strata E, Desmet W, Aquaro GD, Dymarkowski S, Valenti V, Janssens S, Lombardi M, Van de Werf F, L'Abbate A, Bogaert J. Myocardial salvage by CMR correlates with LV remodeling and early ST‐segment resolution in acute myocardial infarction. JACC Cardiovasc Imaging. 2010;3:45–51. [DOI] [PubMed] [Google Scholar]
- 10. Volders PG, Willems IE, Cleutjens JP, Arends JW, Havenith MG, Daemen MJ. Interstitial collagen is increased in the non‐infarcted human myocardium after myocardial infarction. J Mol Cell Cardiol. 1993;25:1317–1323. [DOI] [PubMed] [Google Scholar]
- 11. Tsuda T, Gao E, Evangelisti L, Markova D, Ma X, Chu ML. Post‐ischemic myocardial fibrosis occurs independent of hemodynamic changes. Cardiovasc Res. 2003;59:926–933. [DOI] [PubMed] [Google Scholar]
- 12. Marijianowski MM, Teeling P, Becker AE. Remodeling after myocardial infarction in humans is not associated with interstitial fibrosis of noninfarcted myocardium. J Am Coll Cardiol. 1997;30:76–82. [DOI] [PubMed] [Google Scholar]
- 13. Chan W, Duffy SJ, White DA, Gao XM, Du XJ, Ellims AH, Dart AM, Taylor AJ. Acute left ventricular remodeling following myocardial infarction: coupling of regional healing with remote extracellular matrix expansion. JACC Cardiovasc Imaging. 2012;5:884–893. [DOI] [PubMed] [Google Scholar]
- 14. Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Lindsay M, Watkins S, Hood S, Davie A, Mahrous A, Sattar N, Welsh P, Tzemos N, Radjenovic A, Ford I, Oldroyd KG, Berry C. Pathophysiology of LV remodeling in survivors of STEMI: inflammation, remote myocardium, and prognosis. JACC Cardiovasc Imaging. 2015;8:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub‐clinical myocardial pathology. Eur Heart J. 2012;33:1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kellman P, Wilson JR, Xue H, Bandettini WP, Shanbhag SM, Druey KM, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson. 2012;14:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson. 2012;14:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX; American College of Cardiology F, American Heart Association Task Force on Practice G, American College of Emergency P, Society for Cardiovascular A and Interventions . 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82:E1–E27. [DOI] [PubMed] [Google Scholar]
- 19. Steg PG, James SK, Atar D, Badano LP, Blomstrom‐Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van ‘t Hof A, Widimsky P, Zahger D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 20. Kellman P, Hansen MS. T1‐mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ledesma‐Carbayo MJ, Kellman P, Hsu LY, Arai AE, McVeigh ER. Motion corrected free‐breathing delayed‐enhancement imaging of myocardial infarction using nonrigid registration. J Magn Reson Imaging. 2007;26:184–190. [DOI] [PubMed] [Google Scholar]
- 23. Kellman P, Arai AE. Cardiac imaging techniques for physicians: late enhancement. J Magn Reson Imaging. 2012;36:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arheden H, Saeed M, Higgins CB, Gao DW, Bremerich J, Wyttenbach R, Dae MW, Wendland MF. Measurement of the distribution volume of gadopentetate dimeglumine at echo‐planar MR imaging to quantify myocardial infarction: comparison with 99mTc‐DTPA autoradiography in rats. Radiology. 1999;211:698–708. [DOI] [PubMed] [Google Scholar]
- 25. Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast‐enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. [DOI] [PubMed] [Google Scholar]
- 26. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS; American Heart Association Writing Group on Myocardial S, Registration for Cardiac I . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18:539–542. [PubMed] [Google Scholar]
- 27. Schulz‐Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff‐Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ugander M, Bagi PS, Oki AJ, Chen B, Hsu LY, Aletras AH, Shah S, Greiser A, Kellman P, Arai AE. Myocardial edema as detected by pre‐contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, Raman SV. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. 2011;4:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Obuchowski NA. Nonparametric analysis of clustered ROC curve data. Biometrics. 1997;53:567–578. [PubMed] [Google Scholar]
- 31. Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay M, Mahrous A, Ford I, Tzemos N, Sattar N, Welsh P, Radjenovic A, Oldroyd KG, Berry C. Prognostic significance of infarct core pathology revealed by quantitative non‐contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST‐elevation myocardial infarction survivors. Eur Heart J. 2016;37:1044–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pennell D. Myocardial salvage: retrospection, resolution, and radio waves. Circulation. 2006;113:1821–1823. [DOI] [PubMed] [Google Scholar]
- 33. Botker HE, Kaltoft AK, Pedersen SF, Kim WY. Measuring myocardial salvage. Cardiovasc Res. 2012;94:266–275. [DOI] [PubMed] [Google Scholar]
- 34. Engblom H, Heiberg E, Erlinge D, Jensen SE, Nordrehaug JE, Dubois‐Rande JL, Halvorsen S, Hoffmann P, Koul S, Carlsson M, Atar D, Arheden H. Sample size in clinical cardioprotection trials using myocardial salvage index, infarct size, or biochemical markers as endpoint. J Am Heart Assoc. 2016;4:e002708 doi: 10.1161/JAHA.115.002708. [DOI] [PMC free article] [PubMed] [Google Scholar]


