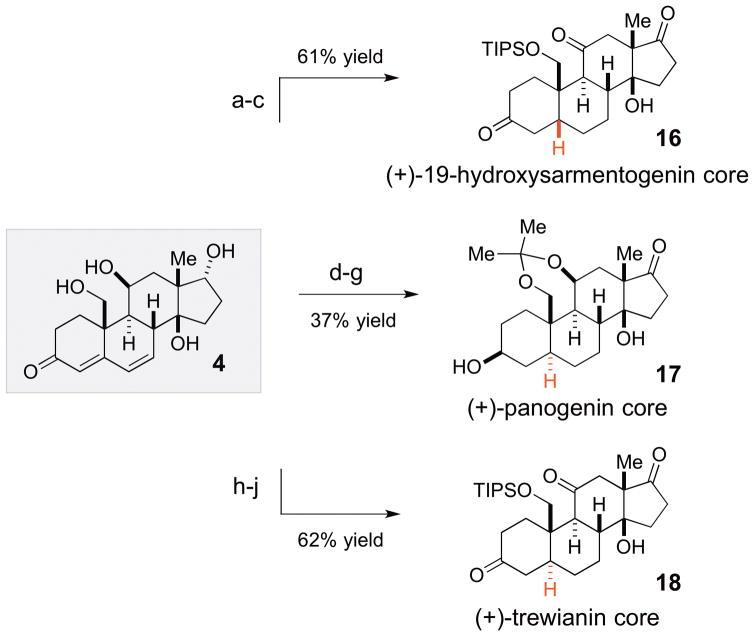
Scheme 4.
Elaboration of intermediate 4 into precursors of 19-hydroxysarmentogenin (2), panogenin (1D) and trewianin (3)a
aReagents and conditions: (a) H2, 10% Pd/C (25% w/w loading), KOH (1% w/v), quinoline (1% v/v), MeOH, 84% yield, >20:1 dr; (b) TIPSCl, ImH, DMF, 6 h, 82% yield; (c) DMP, Py, CH2,Cl2, 2 h, 89% yield; (d) 2,2′-dimethoxypropane, (+)-CSA, DMF, 69% yield; (e) DMP, Py, CH2Cl2, 1.5 h, 90% yield; (f) H2, Pd/C (25% w/w), EtOAc/MeOH/CH2Cl2 (3:1:1), 20 min, 2.5:1 dr; (g) LiAl(OtBu)3H, THF, −78 to −40 °C; 2.5 h, 59% yield, >20:1 dr (2 steps); (h) TIPSCl, ImH, DMF, rt, 6 h, 81% yield; (i) DMP, Py, CH2Cl2, 2 h, 91% yield; (j) H2, Pd/C, MeOH, Py, 4 h; 83% yield, >20:1 dr.