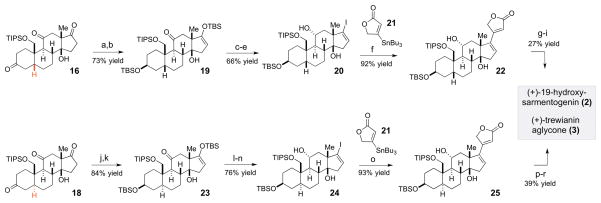
Scheme 5.
Stereodivergent conversion of intermediates 16 and 18 into (+)-19-hydroxysarmentogenin (2) and (+)-trewianin aglycone (3)
aReagents and conditions: (a) K-selectride, THF, −78 to −30 °C, 1.5 h, 77% yield, >20:1 dr; (b) TBSOTf, Et3N, CH2Cl2, −78 to −30 °C, 1.5 h, 95% yield; (c) Li, NH3, THF, −78 °C, 30 min; (d) TBAF, THF, −78 °C, 5 min, 73% yield (2 steps); (e) N2H4•H2O, Et3N, EtOH, 50 °C, 6 h then I2, Et3N, THF, rt, 1 h, 90% yield; (f) 21, Pd(PPh3)4, CuCl, LiCl, DMSO, 50 °C, 1 h, 92% yield; (g) TMSOTf, 2,6-lutidine, CH2Cl2, −78 °C to rt, 2h, then SiO2 (dry), 10 h, 64% yield; (h) H2, Pd/C, EtOAc, 30 min, 2.7:1 dr (β-C17: α–C17); (i) HF in CH3CN/H2O/CH2Cl2, rt, 3 days, 42% yield, 2 steps; (j) LiAlH(OtBu)3, THF, −78 to −40 °C, 4 h, 96% yield, >20:1 dr; (k) TBSOTf, Et3N, CH2Cl2, −78 to −30 °C, 1.5 h, 87% yield; (l) Li, NH3, THF, −78 °C, 30 min; (m) TBAF, THF, −78 °C, 5 min, 91% yield (2 steps); (n) N2H4•H2O, Et3N, EtOH, 50 °C, 6 h then I2, Et3N, THF, rt, 1 h, 83% yield; (o) 21, Pd(PPh3)4, CuCl, LiCl, DMSO, 50 °C, 1 h, 93% yield; (p) TMSOTf, 2,6-lutidine, CH2Cl2, −78 °C to rt, 2h, then SiO2 (dry), 10 h, 79% yield; (q) H2, Pd/C, EtOAc, 30 min; 54% yield of β-C17 and 22% of α-C17; (r) HF in CH3CN/H2O/CH2Cl2, 3 days, 91% yield.