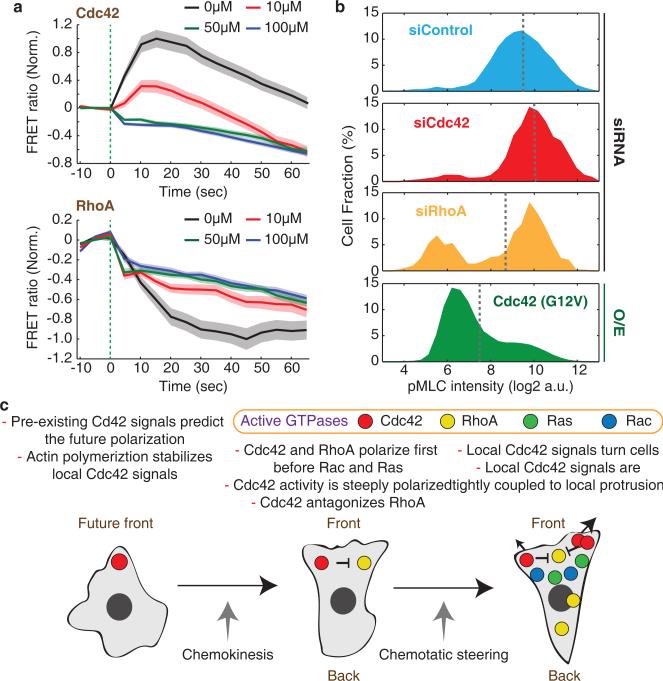
Figure 8.
Cdc42 antagonizes RhoA activity. (a) Effect of the Cdc42 inhibitor ZCL278 on the kinetics of cell-averaged Cdc42 (Top) and RhoA (Bottom) activities in response to chemoattractant photoreleasae. Values were normalized by the initial and maximum activity. Green dotted lines mark the time of chemoattractant release. Error bars indicate ± s.e.m. of n=73 (Cdc42 0μM), n=75 (Cdc42 10μM), n=89 (Cdc42 50μM), n=114 (Cdc42 100μM), n=88 (RhoA 0μM), n=83 (RhoA 10μM), n=104 (RhoA 50μM), and n=130 (RhoA 100μM) cells. Of note, we observed a small but immediate apparent decrease in the observed FRET ratios for both sensors that we believe is an artifact from interaction between UV light and the ZCL278 compound. (b) Histogram of phosphomyosin light chain (pMLC) intensities measured in individual cells by immunofluorescence for control cells and cells perturbed by Cdc42 or RhoA knockdown or expression of a constitutive active Cdc42. Gray dotted lines mark mean pMLC intensities. n=8635 (siControl), n=2419 (siCdc42), n=634 (siRhoA), and n=22201 (Cdc42 (G12V)) cells. (c) Proposed model of GTPase activation for neutrophil polarization. Cdc42 activity has an autocatalytic character and locally inhibits RhoA activity to define and steer the front of the cell. Ras and Rac polarize later and support polarization.