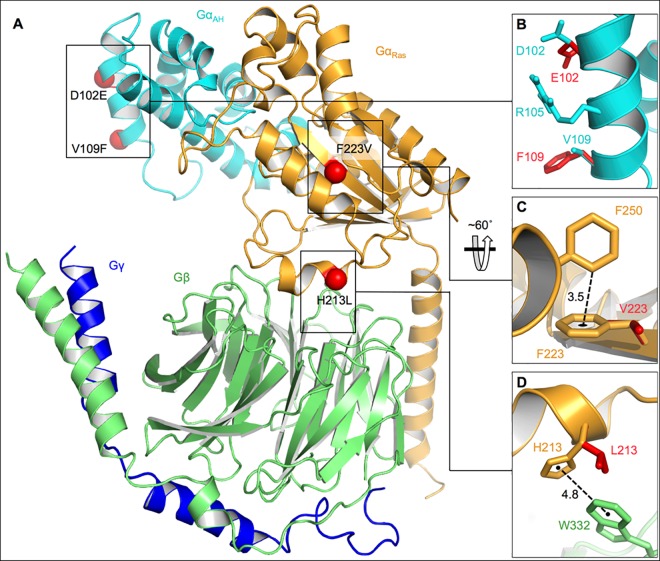
Fig 6. Structural representation of heterotrimeric GNAI3.
A) In the Gαβγ model, two domains of Gα are depicted: the Gα-helical insertion domain (GαAH) is in cyan and the Ras-like GTPase domain (GαRas) is in gold; the Gβ subunit is in green, and the Gγ subunit in blue. Sites of observed amino-acid mutations are indicated with red spheres. B) Magnified representation of mutations D102E and V109F as well as of amino-acid R105. Wild-type side chain carbons are in cyan while mutated residues are in red. C) Magnified representation of the “T-shaped” π-stacking interaction between F223 and F250 in wild-type GNAI3 (gold) that is lacking in the F223V mutant (red). The distance from the ortho carbon on F250 to the centroid of F223 is 3.5 Å, shown with a dotted black line. D) Magnified representation depicting the “parallel displaced” π-stacking interaction between α-H213 (gold) and β-W332 (green) that is absent in the H213L mutant (red). The distance between the centroids of the aromatic rings is 4.8 Å, shown with a dotted black line.