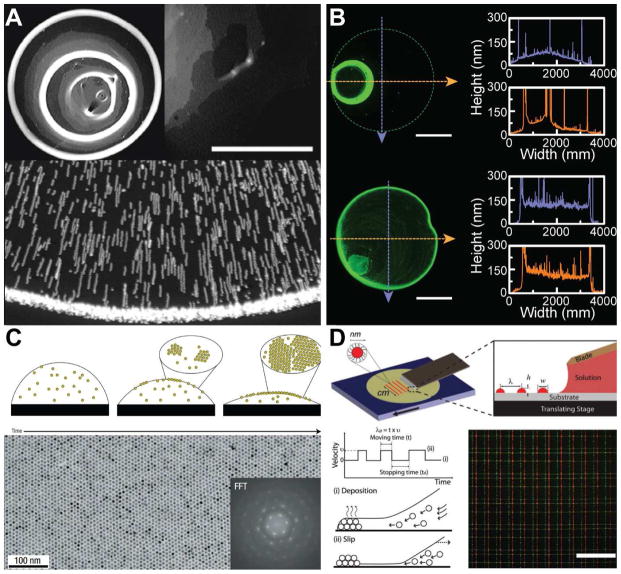
Figure 3.
Microscale patterning of nanoscale inks on a surface. (A) Challenges inherent to assembling particles via convective self-assembly methods. Top left figure shows formation of so-called “coffee-rings,” typically observed when a colloidal suspension droplet dries on a surface. The photograph is of a deposit left by 100 nm microspheres with a volume fraction of 1%. Top right figure shows non-uniformity in the region of the ring, where the grey scale indicates the density of particles with the white color indicating the highest density. Scale bar is 500 μm [124]. Bottom figure show the superimposed exposures that illustrate the motion of the particles toward the edge of the droplet during the drying process [123]. (B) Non-uniformity can be reduced by introducing a co-solvent. Top figure shows the deposition of quantum dots from pure toluene, while bottom figure shows an improvement in the morphology via the introduction of 20% dichlorobenzene [61]. Scale bar is 1 mm. (C) Evaporation kinetics and particle interactions with the liquid-air interface can be tailored to achieve monolayer assembly of nanoparticles. Micrograph shows the monolayer produced by a solution of dodecanethiol-ligated 6 nm gold nanocrystals. Inset shows the fast Fourier transform (FFT) of the image [136]. (D) Arrays of quantum dots are generated via stick-slip motion of the contact line. The features are controlled by the velocity profile of the translation stage. Bottom right figure shows the fluorescent microscopy image of grid patterns of the quantum dots. Scale bar is 200 μm [142]. Reprinted with permission from Refs. [124], [123], [61], [136], [142], respectively. Copyright 2000 American Physical Society, 1997 Nature Publishing Group, 2014 American Chemical Society, 2006 Nature Publishing Group, 2010 John Wiley & Sons.