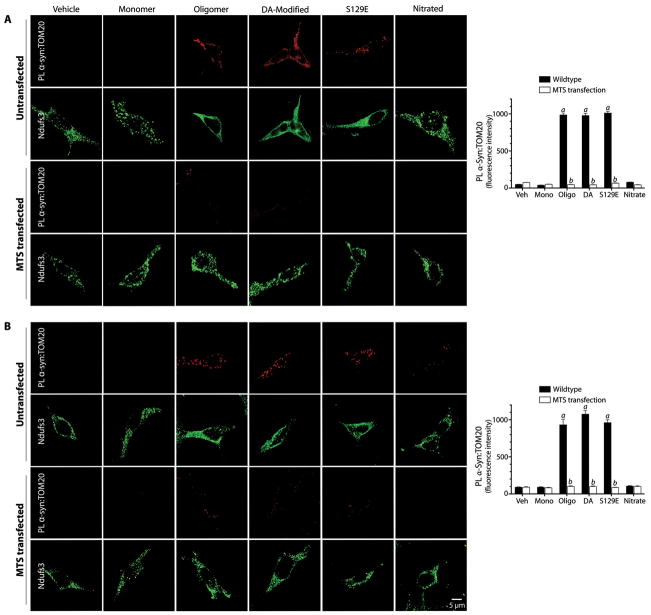
Figure 4.
Proximity ligation of post-translationally modified α-synuclein and TOM20 and Ndufs3 localization in HEK293 cells. (A) In untransfected cells, there is proximity ligation between TOM20 and oligomeric, dopamine-modified and S129E α-synuclein, but not monomeric or nitrated species. This is associated with a cytosolic redistribution of Ndufs3. Fibrillar α-synuclein did not interact with TOM20 (figure S5). When cells were transfected with an MTS expression vector prior to treatment with α-synuclein, the TOM20:α-synuclein interaction was blocked, indicating that the α-synuclein binding site overlaps the MTS binding site on TOM20. MTS transfection also preserved the punctate (mitochondrial) distribution of Ndufs3. (B) When cells were transfected with the MTS expression vector 24h after α-synuclein treatment, the TOM20:α-synuclein interaction was reversed. Bar graphs show quantification of the α-synuclein:TOM20 PL signal in mock transfected (black bars) and MTS-overexpressing cells. At least 100 cells were analyzed for each condition in every independent experiment (N=3). a, p<0.0001 vs vehicle; b, p<0.0001 vs mock transfected; 2-way ANOVA. Scale bar = 5 μm.