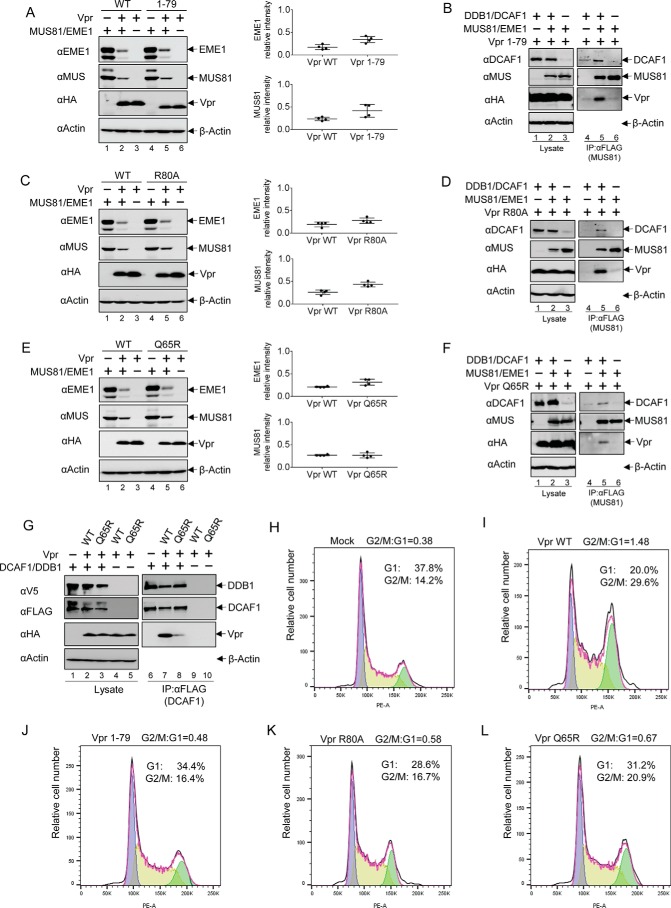
FIGURE 5.
Vpr mutants that lack G2 arrest activity down-regulate MUS81-EME1. A, MUS81 and EME1 plasmids were co-transfected with WT Vpr or a C-terminally truncated Vpr mutant 1–79 plasmid, and empty vector plasmid was added to balance total plasmid in HEK293T cells. After 48 h of transfection, cells were harvested, and lysates were analyzed by immunoblotting. Intensity of bands corresponding to MUS81 and EME1 was quantified from four independent experiments. B, DDB1, DCAF1, MUS81, EME1, and Vpr(1–79)-encoding plasmids, along with empty vector plasmids, were transfected as indicated. FLAG-tagged MUS81 proteins were immunoprecipitated (IP) with anti-FLAG antibody from cell lysates and analyzed with immunoblotting. C, MUS81 and EME1 plasmids were co-transfected with Vpr R80A or Vpr WT plasmids. D, combinations of MUS81, EME1, DDB1, DCAF1, and Vpr R80A were transfected as indicated. Proteins associated with MUS81 were analyzed as described above. E, MUS81 and EME1 plasmids were co-transfected with Vpr Q65R plasmid or Vpr WT plasmid, which were balanced by empty vector plasmid. F, Vpr Q65R plasmids were transiently expressed in cells along with combinations of MUS81, EME1, DDB1, and DCAF1 plasmids. MUS81-associated proteins were analyzed as describe above. G, Vpr WT or Q65R mutant plasmids were co-transfected with DDB1 and DCAF1 plasmids. FLAG-tagged DCAF1 proteins were immunoprecipitated with anti-FLAG antibody from cell lysates, and associated proteins were analyzed by Western blotting. H and J, cell cycle analysis of HEK293T cells transiently transfected with empty vector plasmid (H), Vpr WT (I), Vpr(1–79) (J), Vpr R80A (K), or Vpr Q65R (L) plasmid. The ratio of cells in G2/M relative to those in G1 is indicated.