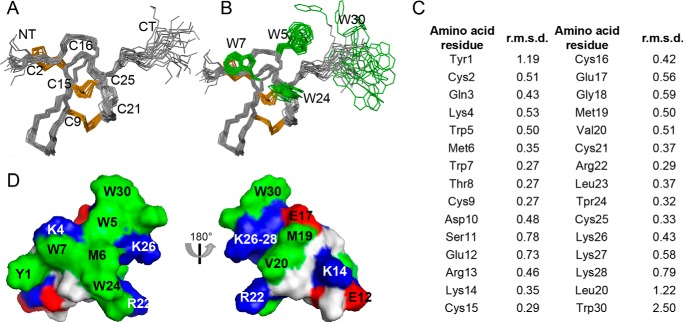
FIGURE 2.
Three-dimensional structure of ProTx-II. A, ensemble of the 20 lowest energy conformations of ProTx-II (PDB code 2N9T) superimposed over the backbone atoms of amino acid residues 2–26. Disulfide bonds are highlighted in orange and the N and C termini are labeled NT and CT, respectively. B, as for A but with the surface-exposed Trp residues shown in green. Note the disordered C-terminal region of ProTx-II, including Trp-30. C, individual amino acid residue r.m.s.d. calculated using molmol version 1.0.7. D, surface representation of ProTx-II highlighting the amphipathic nature of the peptide. Hydrophobic, cationic, and anionic amino acid residues are shown in green, blue, and red, respectively.