FIGURE 20.
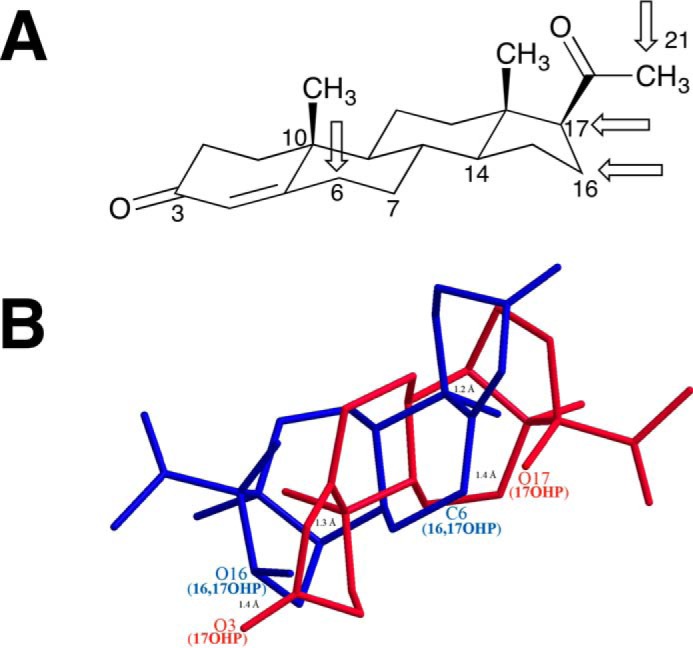
Sites of hydroxylation of progesterone by P450 17A1. A, chair configuration of progesterone, with the four sites of attack indicated by arrows. B, wire diagram of 17α-hydroxyprogesterone (17OHP, red) and 16α,17α-dihydroxyprogesterone (16,17OHP, blue) overlaid, with the latter in an alternative configuration to show the proximity of the C-6 atom of 16α,17α-hydroxyprogesterone with the 17-hydroxy group of 17α-hydroxyprogesterone. The model was made using Chem3D, with a minimum root mean square error of 0.1 and minimum root mean square gradient of 0.01. The C14, C10, and O3 atoms of 17α-hydroxyprogesterone were aligned with the C10, C14, and O16 atoms of 16α,17α-dihydroxyprogesterone, respectively, by displaying the distance measurements of each pair of atoms and then running an overlay minimization calculation. The green lines indicate the pair of atoms that were aligned (after overlay minimization, the distances between C14 of 17-OHP and C10 of 16,17-OHP; C10 of 17-OHP and C14 of 16,17-OHP; and O3 of 17-OHP and O17 of 16,17-OHP were 1.2, 1.3, and 1.4 Å, respectively, and are shown as green lines). The distance between the O17 atom of 17α-hydroxyprogesterone and C6 atom of 16α,17α-dihydroxyprogesterone was 1.4 Å.
