FIGURE 5.
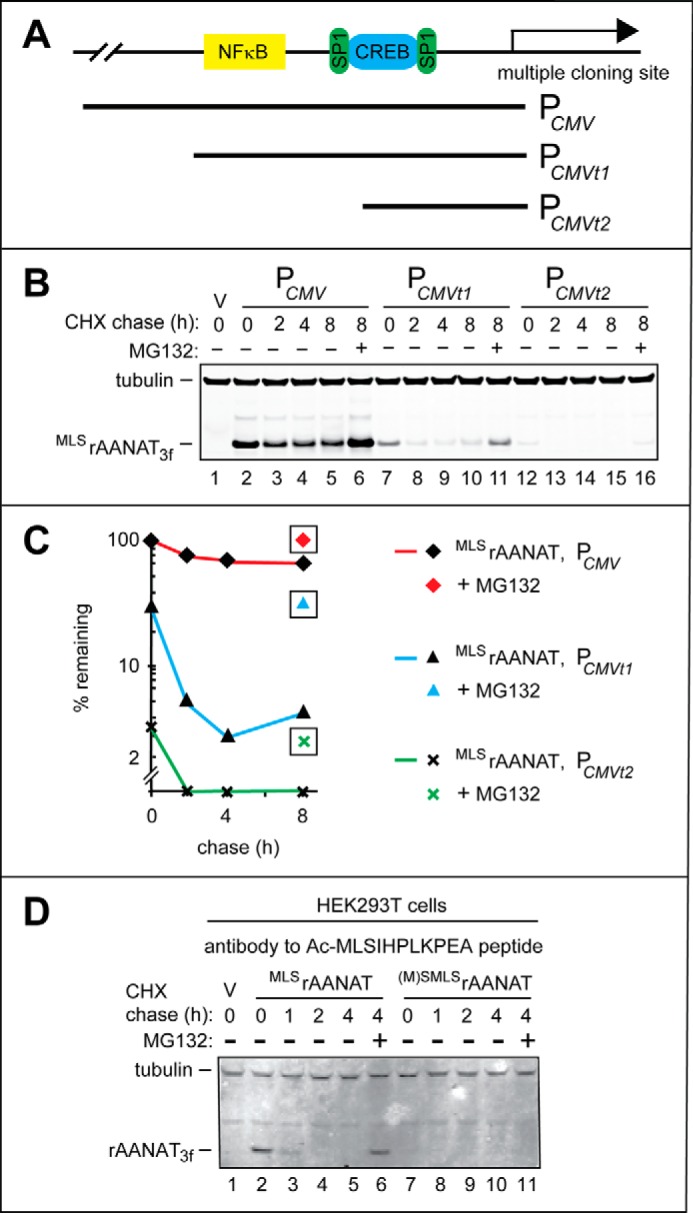
Weakened derivatives of the PCMV promoter in HEK293T cells and degradation assays using antibody to Nt-acetylated rat MLSrAANAT. A, a simplified diagram of the “wild-type” (unmodified) PCMV promoter in the cloning vector, with schematically illustrated binding sites for the NFκB, SP1, and CREB transcriptional regulators. Two 5′-terminal truncations of wild-type PCMV, denoted as PCMVt1 and PCMVt2, are also indicated. B, lane 1, HEK293T cells were transformed with vector (V) alone (control). CHX-chases were performed at 37 °C for the indicated times in HEK293T cells with the wild-type rat MLSrAANAT3f expressed either from the unmodified PCMV promoter (lanes 2–5), from the PCMVt1 promoter (lanes 7–10), or from the PCMVt2 promoter (lanes 12–15). Lanes 6, 10, and 16, same as lanes 5, 9, and 15 except that the MG132 proteasome inhibitor was present during 8-h CHX-chases in each case (see “Experimental Procedures” and “Results”). The bands of tubulin and MLSrAANAT3f are indicated on the left. C, quantification of data in B. Symbols shown separately and framed in black squares correspond to altered (increased) levels of test proteins in the presence of MG132. All quantified CHX-chase assays were carried out at least three times and yielded results within 10% of the data shown. D, immunoblotting with affinity-purified antibody to Nt-acetylated MLSrAANAT (see “Experimental Procedures”). Lane 1, HEK293T cells were transformed with vector (V) alone (control) bearing the (weakened) PCMVt1 promoter. CHX-chases were performed at 30 °C for the indicated times in HEK293T cells with the wild-type rat MLSrAANAT3f (lanes 2–5) and its mutant (M)SMLSrAANAT3f (lanes 7–10). Lanes 6 and 11, same as lanes 5 and 10 except that the MG132 proteasome inhibitor was present during 4-h CHX-chases in each case (see “Experimental Procedures”). The bands of rAANAT and tubulin (the latter a loading control) are indicated on the left.
