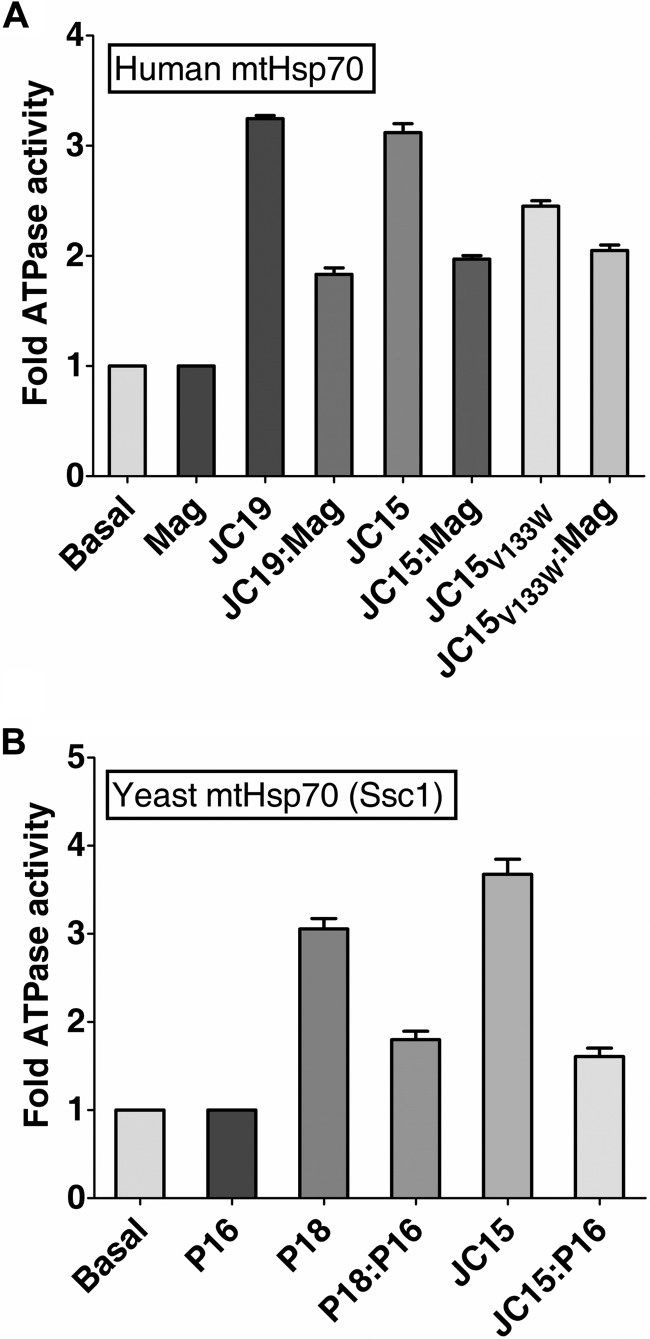
FIGURE 5.
Relative ability of the J-proteins to stimulate mtHsp70 ATPase activity. A, comparative analysis of stimulation of human mtHsp70 ATPase activity by J-protein paralogs. mtHsp70·[α-32P]ATP complex was incubated with a 2-fold excess of either Magmas (Mag) alone, JC19 alone, Magmas·JC19 subcomplex, JC15 alone, or Magmas·JC15 subcomplex, and the rate of hydrolysis was measured as the function of time at 23 °C. The basal rate of ATP hydrolysis by mtHsp70 alone was set to 1. The rate of conversion to ADP was measured and represented as -fold stimulation over the basal rate. n = 3, p (two tailed) < 0.001). B, effect of Pam16·JC15 subcomplex on Ssc1 ATPase activity. Ssc1·[α-32P]ATP complex was incubated with a 2-fold excess of either Pam16 (P16) alone, JC15 alone, or Pam16·JC15 subcomplex, and the rate of hydrolysis was measured as a function of time at 23 °C. Pam18 (P18) was used as a positive control. The basal rate of ATP hydrolysis by Ssc1 alone was set to 1.