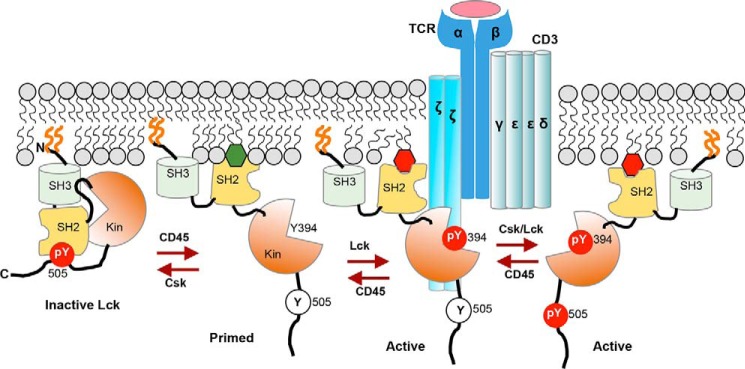
FIGURE 8.
Hypothetical model for spatiotemporal coordination of protein interaction and signaling activity of Lck by PM lipids during T cell activation. Lck is PM-localized by N-terminal lipidation (orange lines) but remains inactive due to intramolecular tethering of its catalytic domain (Kin) by SH2 and SH3 domains. Upon TCR activation, dephosphorylation of Tyr-505 by CD45 unleashes Lck-SH2 from the catalytic domain and allows its interaction with PM lipids, including PI4,5P2 (green hexagon) and autophosphorylation on Tyr-394 for full enzymatic activation. Lipid-Lck-SH2 binding is crucial for spatiotemporally specific interaction of Lck with proteins in the activated TCR-CD3 complex, including the ζ chain, and for preventing inactivation of another active species, Tyr(P)-394/Tyr(P)-505, by Tyr(P)-505-Lck-SH2 intramolecular tethering. Both Lck-Tyr(P)-394 and Lck-Tyr(P)-394/Tyr(P)-505 can effectively phosphorylate ITAMs in the CD3 and ζ chains to initiate the TCR signaling. PIP3 (red hexagon), which is subsequently produced by PI3K and accumulated near the TCR-CD3 complex, allows sustained activation of Lck via its tight interaction with Lck-SH2.