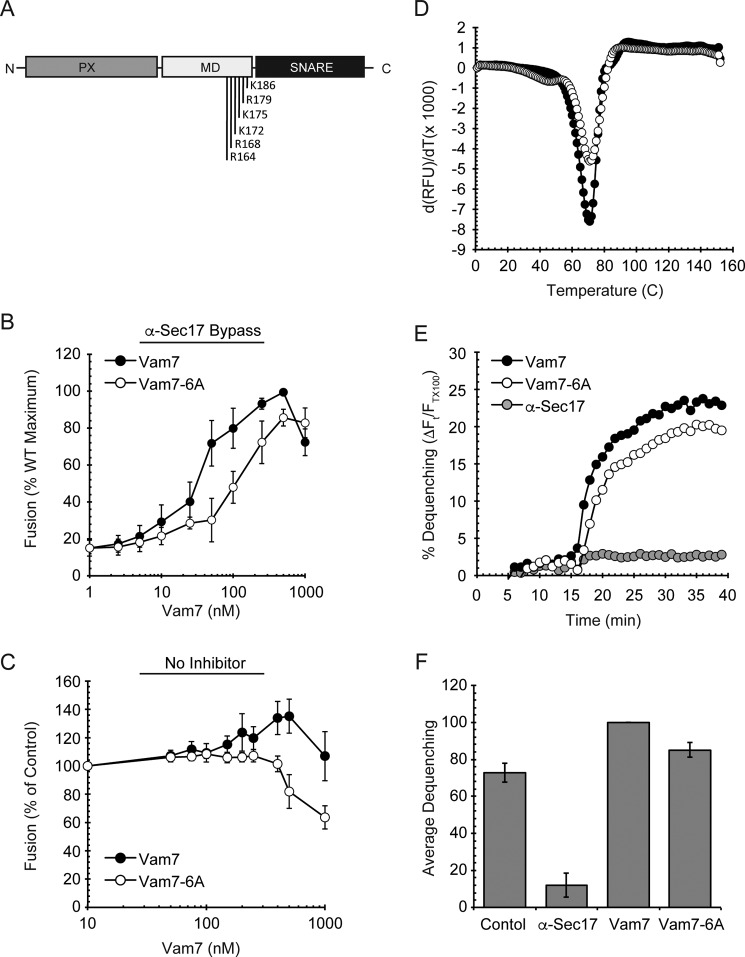
FIGURE 1.
Vam7-6A requires higher concentrations to reach Vam7 fusion levels. A, schematic representation of Vam7 and its polybasic region in the middle domain (MD). B, vacuole fusion reactions were performed using vacuoles from BJ3505 and DKY6281. Fusion reactions were incubated with 60 μg/ml α-Sec17 IgG for 10 min on ice followed by the addition of recombinant wild type Vam7 or Vam7-6A at the indicated concentrations for 5 min on ice. Fusion reactions were incubated at 27 °C for 90 min and assayed for Pho8 activity. C, standard fusion reactions (no inhibitors) were incubated with Vam7 or Vam7-6A at the indicated concentrations and incubated at 27 °C for 90 min to test fusion. D, differential scanning fluorimetry. Vam7 and Vam7-6A were incubated with SYPRO orange. Samples were equilibrated for 30 min before starting the melting curve between 20 °C and 95 °C. SYPRO orange fluorescence was measured at each temperature (λex = 490 nm, λem = 560 nm). Shown are the first derivatives of the fluorescence data to depict Tm for each sample. E, lipid Mixing (hemifusion) assays were performed using WT vacuoles inhibited with anti-Sec17 IgG at 4 °C. Reactions were transferred from ice to 27 °C and incubated for 5 min before the addition of ATP regenerating system. After an additional 10-min incubation wild type Vam7 or Vam7-6A was added to the indicated reactions. An increase in fluorescence occurred when the outer leaflet of vacuoles mixed during hemifusion. Shown is a representative of three trials. F, the average lipid dequenching at 35 min after the addition of ATP. Error bars indicate S.E. (n = 3).