FIGURE 6.
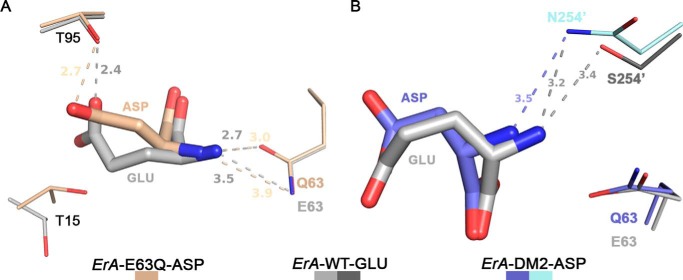
Structural basis for selective discrimination against Gln. A, docking of Glu from the ErA-WT-Glu structure (light and dark gray) into the ErA-E63Q·Asp active site (beige). To fit in the active site, the extra methylene group of Glu (versus Asp) requires the larger amino acid to shift in the direction of Glu-63. The substitution of Glu-63 by a glutamine introduces a H-bond donor (the amide nitrogen atom) in proximity to the Glu α-amino group (also a H-bond donor). It is this proximity of the H-bond donor moieties that selects against Gln. Glu (and pertaining to Gln) cannot shift away as the opposite side of the substrate is anchored by Thr-95. For the smaller amino acid Asn, this negative constellation is mitigated by the larger distance to Gln-63. B, docking of Glu from the ErA-WT-Glu structure into the ErA-DM2·Asp active site (plum and cyan). Like the E63Q mutation, the S254N mutation introduces a H-bond donor (the amide nitrogen atom of the Asn-254′ side chain) in proximity to the substrate α-amino group. Glu (and pertaining to Gln), due to the extra methylene group, cannot avoid this negative interaction.