FIGURE 5.
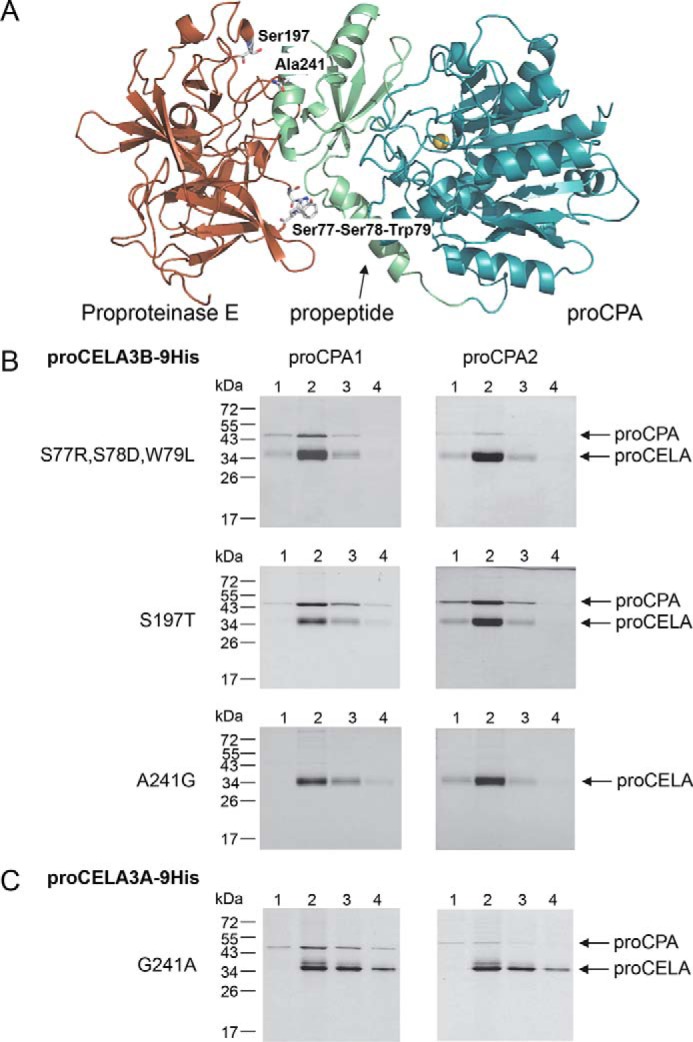
Structural determinants of defective proCELA3A binding to proCPA1 and proCPA2. A, the positions of amino acid differences between proCELA3A and proCELA3B were mapped to the binding interface between bovine proCPA and proproteinase E. For clarity, chymotrypsinogen C was omitted from the ternary complex (Protein Data Bank file 1PYT). Amino acid residues at or near the interface are indicated. B, binding of proCELA3B triple mutant S77R,S78D,W79L and single mutants S197T and A241G to proCPA1 and proCPA2. C, binding of proCELA3A mutant G241A to proCPA1 and proCPA2. Qualitative binding experiments using conditioned media with His-tagged proelastases and non-tagged procarboxypeptidases were carried out as described under ”Experimental Procedures.“ The numbers above the lanes indicate the fractions eluted from the nickel column. Representative gel pictures of two experiments are shown.
