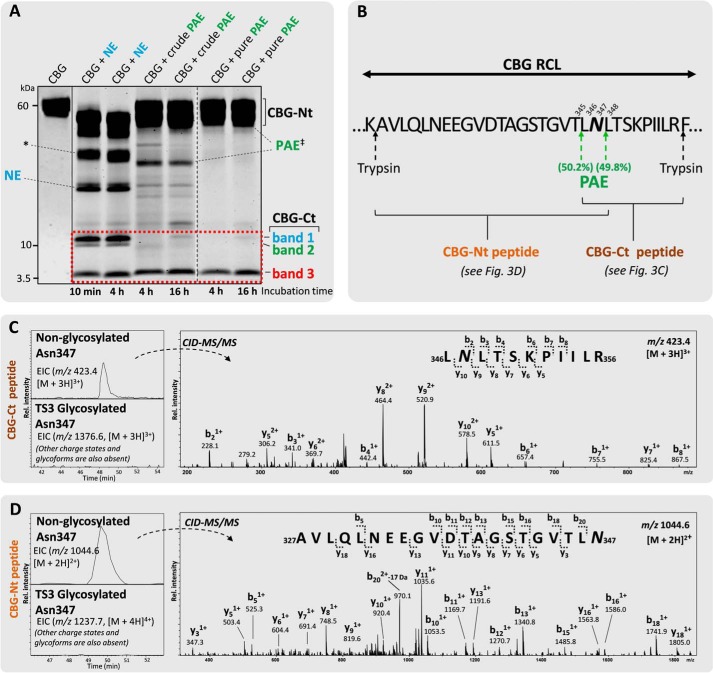
FIGURE 3.
Glycan occupancy of Asn347 abolishes the PAE-induced RCL cleavage. A, unlike NE-digested (light blue) CBG (two penultimate left lanes), crudely isolated and pure PAE (green) generated two relatively homogeneous complementary CBG fragments (i.e. a single CBG-Ct (band 3, 5 kDa) and a CBG-Nt (50–55 kDa) fragment under extended incubation (4 and 16 h, right lanes) as evaluated by SDS-PAGE. Undigested native CBG is shown in the far left lane. Broken line, non-neighboring gel lanes from the same gel. Solid line, gel lanes from different gels. B, amino acid residues of the exposed RCL of CBG in the vicinity of the observed PAE cleavage sites (green arrows). The CBG-Nt (gold) and CBG-Ct (brown) peptides covering the Asn347 glycosylation site are shown. C, the presence of 346LNLTSKPIILR356 and 348LTSKPIILR356 (data for the latter not shown) in an Asn347-non-glycosylated form (top EIC, left) and the absence of their corresponding Asn347-glycosylated forms (bottom EIC, left; as exemplified by the absence of an EIC signal for the TS3 Asn347 glycopeptide) in the CBG-Ct fragment was confirmed using CID-MS/MS (right). D, peptides of the complementary CBG-Nt fragments (i.e. 327AVLQLNEEGVDTAGSTGVTLN347 and 327AVLQLNEEGVDTAGSTGVT345 (data for the latter not shown) were also exclusively identified in their non-glycosylated state by LC-MS/MS. No EIC signals were detected in any charge states for any glycoforms of either the CBG-Nt or the CBG-Ct peptides. *, CBG-Nt fragments occurring after extended digestion of CBG with NE that were not further investigated in this study. ‡, crude PAE migrated around 33 kDa as validated by LC-MS/MS analysis, whereas the commercial form of PAE (“pure PAE”) was observed in separate experiments to co-migrate with human CBG around 55–60 kDa (data not shown).