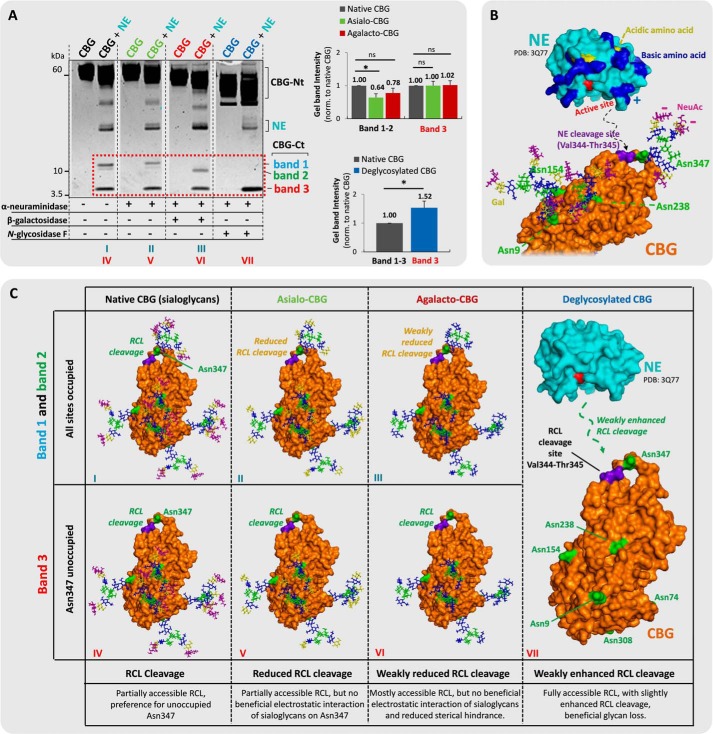
FIGURE 5.
Sialylation of Asn347 glycans, not of glycans from other CBG sites, is beneficial for NE-based RCL cleavage. A, incubations of (from left) undigested native CBG (black), NE-digested native CBG, undigested asialo-CBG (green), NE-digested asialo-CBG, undigested agalacto-CBG (red), NE-digested agalacto-CBG, undigested deglycosylated CBG (blue), and NE-digested deglycosylated CBG. Comparisons of the relative gel band intensities (red box) were performed. Broken line, non-neighboring gel lanes from the same gel. B, the three-dimensional structures of NE (PDB code 3Q77) and RCL uncleaved CBG (homology model based on uncleaved human TBG, PDB code 2CEO). CBG is presented with its most abundant glycoforms of the three sites located in proximity to the RCL (i.e. Asn347 displays TS3, and Asn154 and Asn238 both display BS2). The color coding of the monosaccharide residues and amino acid residues is generally as described in the legend to Fig. 4, the NE cleavage site (Val344-Thr345) on the RCL of CBG is in purple. The proposed electrostatic interactions and protein docking to the RCL of CBG are schematically illustrated with broken lines. C, proposed interactions of NE and the glycosylated variants of CBG, which may explain the differential RCL cleavage observed in Fig. 5A. *, p < 0.05; ns, not significant. Data points are represented as mean ± S.E. (error bars), n = 3.