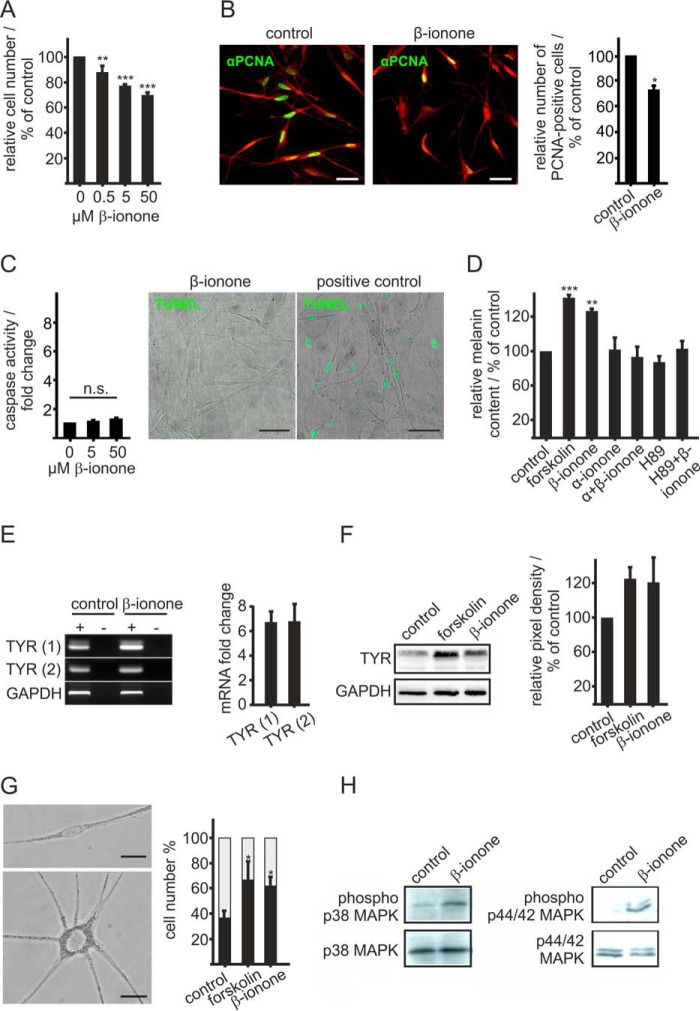
FIGURE 2.
Activation of OR51E2 promotes differentiation. A, β-ionone stimulation affects melanocytes proliferation. Proliferation of melanocytes after treatment with increasing concentrations of β-ionone for 6 days compared with control conditions. The relative cell number was determined by measurement of the DNA content. The data are shown as the mean of three independent experiments with five technical replicates and were normalized to the cell number in control experiments, and significance was calculated by Student's t test referring to cell number of untreated control cells (**, p < 0.01; ***, p < 0.001). B, immunocytochemical staining using an antibody against PCNA reveals significantly reduced numbers of proliferating cells after stimulation with β-ionone compared with control cells. Left panel, immunofluorescence confocal micrographs of melanocytes labeled with a PCNA-specific antibody (green) and Alexa Fluor® 546 phalloidin (red). Melanocytes were treated with 50 μm β-ionone or solvent only (control) for 6 days. The bar indicates 50 μm. Right panel, the data are shown as the mean of four independent experiments each with 500 quantified cells that were normalized to the cell number in control experiments. Significance was calculated by Student's t test referring to cell number of untreated control cells (*, p < 0.05). C, β-ionone stimulation had no significant effect on melanocyte apoptosis. Left panel, induction of apoptotic pathways was assessed by the Caspase-Glo 3/7 assay. Significance was calculated by Student's t test referring to cells treated with the solvent only. Right panel, representative photomicrographs of melanocytes stained by TUNEL assay after treatment with 50 μm β-ionone for 72 h. DNAse I treatment (20 min) served as the positive control for the detection of apoptosis. n.s., not significant. D, β-ionone stimulation induces melanogenesis. Melanin content was measured 72 h after β-ionone treatment (50 μm). The β-ionone-induced effect on pigmentation was suppressed by co-stimulation with α-ionone (200 μm) or prestimulation with H89 (10 μm) for 2 h. The data are the average of the results from three biological replicates each performed in triplicate and were normalized to the melanin content in solvent-only-treated cells (0.1% DMSO, control). Forskolin (20 μm) served as a positive control. Error bars indicate the S.E. Significance was calculated by Student's t test referring to the melanin content in control cells. E, RT-qPCR quantification of tyrosinase gene expression in melanocytes stimulated for 6 h with β-ionone (50 μm), forskolin (20 μm), and solvent only (0.1% DMSO, control). Right panel, gel electrophoresis of tyrosinase amplicons using two different primer pairs (1 and 2) from melanocyte cDNA (+) and no reverse transcriptase controls (−). Amplification of GAPDH served as internal control. Left panel, relative tyrosinase gene expression of β-ionone-stimulated melanocytes using two different primer pairs (1 and 2). Tyrosinase mRNA levels were normalized by GAPDH in each experiment, and changes in mRNA expression were calculated compared with control cells. Bars represent the mean of three experiments, each performed in triplicate. Error bars represent the S.E. F, right panel, Western blotting analysis reveals an β-ionone-induced increase in tyrosinase protein. Cells were stimulated with β-ionone (50 μm) and forskolin (20 μm, positive control) for 72 h. Detection of GAPDH served as the internal standard. Left panel: quantification of tyrosinase signal intensities. Bars represent the mean of four independent experiments; tyrosinase signal intensities were normalized by GAPDH in the same experiment. G, left panel, phase-contrast image of an undifferentiated and differentiated melanocyte in culture. Undifferentiated melanocytes are bipolar, less pigmented, and have small cell bodies, whereas differentiated melanocytes exhibited an increased size of the cell body, increased pigmentation, and multiple dendrites. The bar indicates 10 μm. Right panel, exposure to β-ionone induced melanocyte dendritogenesis. Cells were cultured for 6 days in basal medium containing β-ionone (50 μm) and 0.1% DMSO as negative control. Forskolin (20 μm) served as a positive control. Undifferentiated (gray part of the bar) and differentiated cells (black part of the bar) were counted and are shown as the percentage of total cells. The data are the mean of three independent experiments each with 500 cells quantified. Statistics were performed by Student's t test, referring to the cell number of control cells (*, p < 0.05). H, activation of OR51E2 resulted in phosphorylation of p38 MAPK and p44/42 MAPK (ERK1/2) as shown by Western blotting analyses. Melanocytes were stimulated with 50 μm β-ionone for 30 min. Detection of p38 MAPK and ERK total protein is shown to control loaded protein amounts.