FIGURE 4.
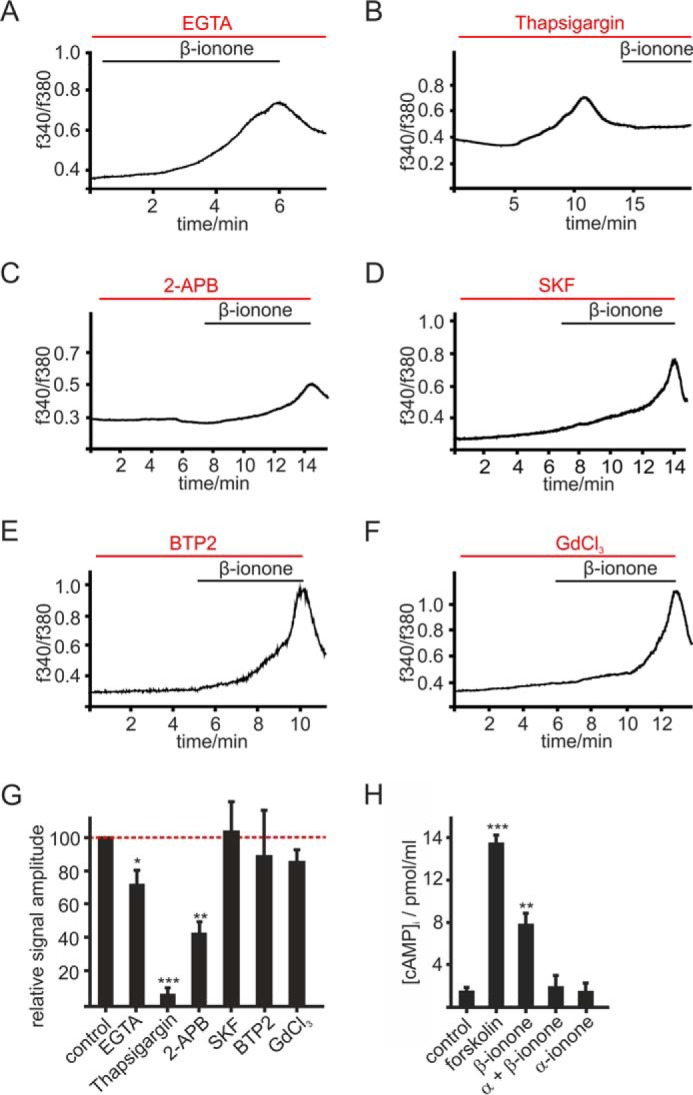
OR51E2 signaling in melanocytes. A–F, representative recordings of Fura-2-loaded melanocytes. Cytosolic Ca2+ concentration is monitored as integrated fluorescence ratio as a function of time. A, Ringer's solution containing 50 μm EGTA decreases the β-ionone (50 μm) induced response. Pretreatment with thapsigargin (10 μm) (B) or 2-APB (25 μm) (C) reduced the β-ionone (50 μm)-induced Ca2+ signals in melanocytes. SKF 96365 (SKF; 10 μm) (D), BTP2 (20 μm) (E), and GdCl3 (100 μm) (F) did not influence the β-ionone (50 μm)-induced Ca2+ response in melanocytes. G, quantification of β-ionone-induced Ca2+ signals in blocker experiments relative to control measurements (β-ionone only) or quantification of Ca2+ signal amplitudes in Ca2+ free conditions (+50 μm EGTA), normalized to the melanocyte responses measured in Ca2+ containing buffer (control). Data significance was calculated using Student's t test referring to the β-ionone induced Ca2+ signal in controls. The data are shown as the means ± S.E. (n ≥ 65 cells) (*, p < 0.05; **, p < 0.01; ***, p < 0.001). H, OR51E2 signaling involves activation of adenylate cyclases. β-Ionone (50 μm, 30 min) stimulation resulted in a 4-fold increase in intracellular cAMP, which was suppressed by co-stimulation with α-ionone (200 μm). Stimulation of melanocytes by α-ionone alone did not increase intracellular cAMP. Forskolin (20 μm) served as a positive control. Experiments were performed as duplicates in four independent experiments. Significance was calculated by Student's t test referring to the intracellular cAMP concentration in control cells. Error bars represent the S.E. (**, p < 0.01; ***, p < 0.001).
