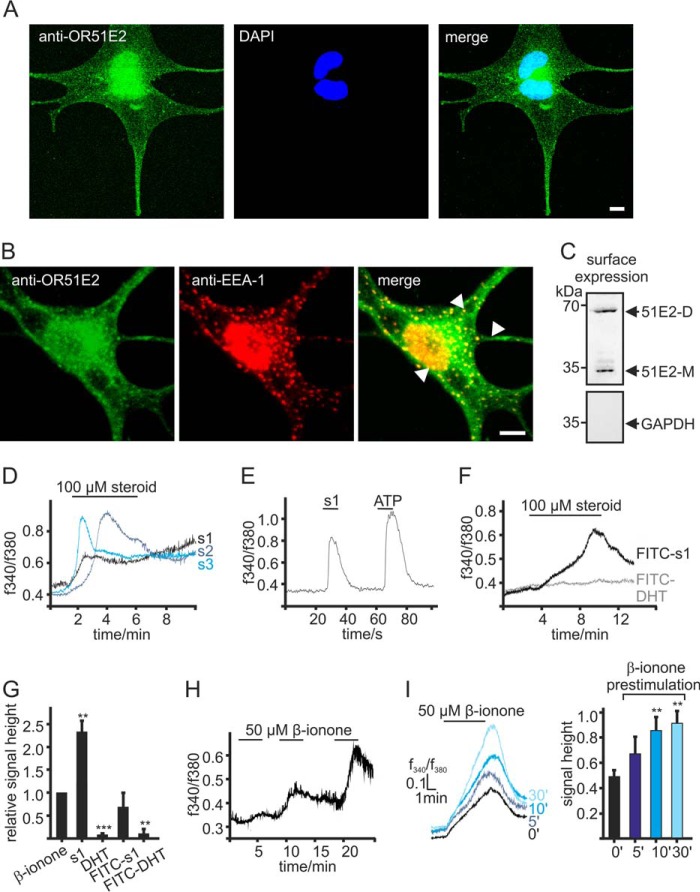
FIGURE 5.
Intracellular localization of OR51E2. A, immunofluorescence confocal micrographs of a melanocyte labeled with an OR51E2-specific antibody (green) and DAPI to visualize the nucleus (blue). Intracellular, vesicular staining of OR51E2 can be observed. The bar indicates 5 μm. B, immunofluorescence confocal micrographs of a melanocyte co-labeled with an OR51E2-specific antibody (green) and an antibody to EAA-1 (red). Co-staining of cellular organelles appears in yellow in the merged image (arrows). The bar indicates 5 μm. C, surface localization of OR51E2 in melanocytes verified by surface biotinylation and detection by Western blotting. A representative Western blotting of biotinylated membrane is shown (n = 3 independent experiments). GAPDH served to control of purification of cell surface proteins only. D, Ca2+ responses of melanocytes to steroid ligands of OR51E2. Shown are representative Ca2+ imaging traces of melanocytes stimulated with the OR51E2 steroid ligands 6-dehydrotestosterone-(s1,2),4,6-androstadien-17α-ol-3-one (s2, 4) and 1,4,6-androstadien-3,17-dione (s3, 5). Ca2+ signals are displayed as integrated fluorescence ratio as a function of time. Steroids were applied for 5 min. E, heterologously expressed OR51E2 responds to FITC-tagged 6-dehydrotestosterone. Shown is a representative Ca2+ imaging trace of a Hana3a cell transiently expressing OR51E2. ATP (20 μm, 2 s) served to control the ability of the cells to produce Gq-mediated Ca2+ signals. The integrated fluorescence ratio (f340/f380) of the Fura-2 loaded cell is shown as a function of time. FITC-6-dehydrotestosterone (100 μm) was applied for 10 s. F, representative Ca2+ imaging recording of melanocytes treated with FITC-steroids (100 μm) for 5 min. Only application of FITC-6-dehydrotestosterone (FITC-s1) induced a Ca2+ signal in the cells; application of FITC-DHT showed no effect on cytosolic Ca2+ levels. G, quantification of cytosolic Ca2+ signal amplitudes upon stimulation of melanocytes with β-ionone (50 μm), 6-dehydrotestosterone (s1, 100 μm), dihydrotestosterone (DHT, 100 μm), FITC-6-dehydrotestosterone (FITC-s1, 100 μm), and FITC-DHT (100 μm). Signal amplitudes were quantified and normalized by the β-ionone-induced maximal signal amplitudes. Three independent experiments (each with >70 cells) were performed in quadruplicates for each steroid, and data are shown as the mean. Significance was calculated by Student's t test for each sample group referring to the β-ionone-induced signal amplitude. Error bars represent the S.E. (**, p < 0.01; ***, p < 0.001). H, repetitive stimulation with β-ionone evoked increasing Ca2+ signals. Representative Ca2+ imaging trace of a Fura-2-loaded melanocyte. β-Ionone (50 μm) was applied for 5 min in each application. I, left panel, different durations of pretreatment with 50 μm β-ionone resulted in increased responses to consecutive stimulation with β-ionone. Shown are representative Ca2+ imaging traces of Fura-2-loaded primary melanocytes; β-ionone (50 μm) was consecutively applied for 5 min. Right panel, quantification of signal amplitudes in melanocytes pretreated with β-ionone (50 μm) for different durations; bars represent the mean normalized to control amplitudes (from cells not pretreated). Three independent experiments were performed for each prestimulation, each with >50 cells. Significance was calculated by Student's t test for each sample group referring to the β-ionone induced Ca2+ signal in control cells. Error bars represent the S.E. (**, p < 0.01).