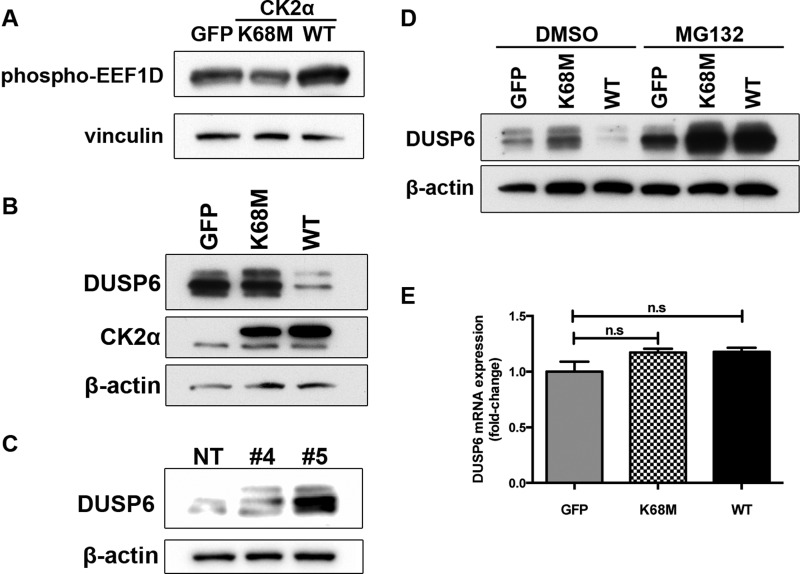
FIGURE 5.
CK2α decreases protein stability of the ERK phosphatase DUSP6 in a kinase-dependent manner. A, phosphorylation of the CK2α substrate EEF1D upon expression of WT or kinase-inactive (K68M) CK2α was detected by Western blotting with a phospho-EEF1D antibody. Levels of endogenous DUSP6 protein were determined by Western blotting of lysates from A375 cells ectopically expressing CK2α(WT) or CK2α(K68M) (B) or from A375 cells depleted of endogenous CK2α by two different shRNAs (same lysates as shown in Fig. 3A) (C). D, to determine whether CK2α regulates DUSP6 protein stability, the same cells as in B were immunoblotted for DUSP6 protein after treatment for 6 h with either the proteasome inhibitor MG132 (10 μm) or DMSO vehicle control. E, to determine whether CK2α also regulates DUSP6 at the transcriptional level, quantitative RT-PCR analysis of DUSP6 mRNA levels was done on cells expressing CK2α(WT) or kinase-inactive CK2α(K68M). Results are presented as means ± S.E. (n = 3). Error bars represent S.E. n.s., not significant.