Brief Summary
We describe a patient with two recent episodes of tenofovir disoproxil fumarate (TDF)-associated acute kidney injury and six-class drug-resistant HIV infection who achieved and maintained viral suppression without worsening kidney function on a regimen including tenofovir alafenamide (TAF) through 48 weeks of therapy. The safety and efficacy of TAF in patients with TDF-associated renal tubulopathy and multiple drug resistance HIV has not yet been described. TAF may represent a useful option to maximally suppress HIV in patients with these complications.
Despite the success of potent combination antiretroviral therapy (ART), there is still a small but notable subset of patients who harbor HIV strains resistant to all antiretroviral drug classes and fail to achieve viral suppression. Some of these patients may have initiated on ART with sequential monotherapy and were treated with drugs that were less potent, more toxic, and had difficult dosing requirements that impacted adherence. This may lead to the accumulation of multiple drug class resistance leading to very limited treatment options.
Tenofovir disoproxil fumarate (TDF) is a nucleotide reverse transcriptase inhibitor that remains an important agent for treatment-experienced patients. However, long-term use of TDF has been associated with renal tubulopathy and decreased bone mineral density (1-3). Moreover, TDF-associated resistance may emerge after long-term suboptimal therapy. Tenofovir alafenamide (TAF) is a novel, investigational prodrug of tenofovir (TFV) that results in lower systemic TFV exposure and greater intracellular TFV concentration after oral administration compared to TDF (4). TAF has demonstrated potent antiviral activities when used in treatment-naïve patients in Phase 3 trials (5), and may alleviate renal and bone toxicities caused by TDF (6). Its role in patients with NRTI-resistance has not been explored. Dolutegravir is a second-generation HIV integrase strand transfer inhibitor with good activity against some HIV strains resistant to raltegravir and elvitegravir (7). We report a case of successful viral suppression with a TAF and dolutegravir-containing regimen in a patient with extensive multi-antiretroviral drug class resistance, with two prior episodes of acute kidney injury (AKI) while receiving TDF.
Case
The patient is a 54-year-old Caucasian man who was diagnosed with HIV-1 infection and Pneumocystis pneumonia in 1995. His CD4+ count was 16 cells/mm3. He underwent zidovudine monotherapy followed by dual NRTI therapy, and then combinations of at least three drugs. Over subsequent years, he achieved an HIV RNA level <50 copies/mL on only one occasion and had been treated with all approved antiretroviral drugs except dolutegravir, maraviroc, delavirdine and rilpivirine. His CD4+ count remained <200 cells/mm3 and <50 cells/mm3 since 2007. His medical history was notable for ART-related complications including peripheral neuropathy, severe lipodystrophy, and dyslipidemia. His comorbidities included basal and squamous cell carcinomas, hypertension, and obstructive sleep apnea. His concomitant medications included SMX/TMP, azithromycin, rosuvastatin, lisinopril, hydrochlorothiazide, temazepam, lorazepam, venlafaxine, testosterone, omega-3 fatty acids, and milk thistle. His physical examination was most notable for a large neck, small buffalo hump, central obesity and thin extremities.
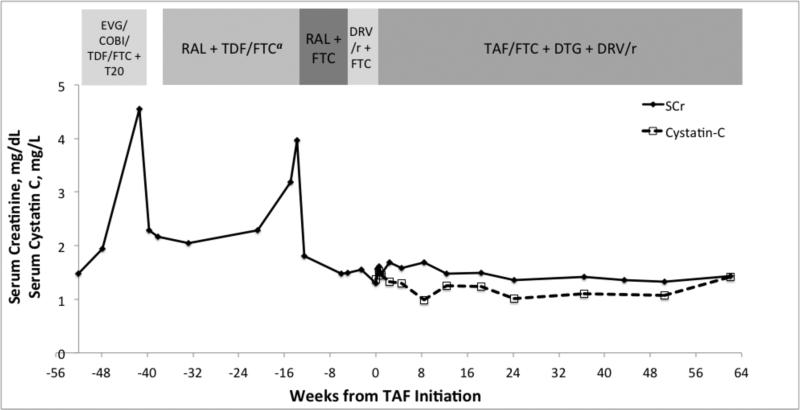
In October 2013, two months after starting a regimen consisting of enfuvirtide and elvitegravir/cobicistat/TDF/emtricitabine (Figure 1), his serum creatinine increased from 1.47 mg/dL (130 μmol/L) to 4.55 mg/dL (402 μmol/L). Correspondingly, his estimated glomerular filtration rate (eGFR) by the CKD-EPI creatinine equation fell from 53 mL/min/1.73 m2 to 14 mL/min/1.73 m2. In the setting of presumed iatrogenic kidney injury, his ART and sulfamethoxazole/trimethoprim (SMX/TMP) were discontinued, which resulted in an improvement in SCr to 2.16 mg/dL (191 μmol/L, eGFR 34 mL/min/1.73 m2) in one month. He then restarted SMX/TMP and ART with raltegravir and renal dose-adjusted TDF 300 mg and emtricitabine 200 mg each taken every other day.
Figure 1.
Antiretroviral regimens, serum creatinine, and cystatin-C prior to and after initiating a salvage regimen with tenofovir alafenamide
COBI = cobicistat, DRV/r = ritonavir-boosted darunavir, DTG = dolutegravir, EVG = elvitegravir, FTC = emtricitabine, RAL = raltegravir, SCr = serum creatinine, TAF = tenofovir alafenamide, TDF = tenofovir disoproxil fumarate, T20 = enfuvirtide
In April 2014, he presented to the National Institutes of Health for enrollment in a protocol aiming to manage patients with ART failure (ClinicalTrials.gov identifier NCT01976715). His laboratory results at presentation revealed SCr of 3.18 mg/dL (281 μmol/L, eGFR 21 mL/min/1.73 m2), CD4 count of 21 cells/mm3 (1%), and HIV RNA of 10,748 copies/mL. Due to his rising SCr, TDF, SMX/TMP, hydrochlorothiazide and lisinopril were discontinued, he received intravenous hydration, and his study enrollment was deferred. A summary of the genotypic and phenotypic resistance testing from this visit (Table 1) revealed resistance to all approved antiretroviral drugs except for sensitivity to dolutegravir (phenotypic IC50 fold-change 2.02) and partial sensitivity to TDF (2.84 fold-change). Sanger genotyping revealed NRTI mutations M184V, M41L, L210W, and T215Y, integrase mutation N155H and extensive PI resistance mutations including I47V, I54M, I84V and L90M. Phenotype reports showed resistance to all protease inhibitors, including darunavir (289 fold-change) and non-nucleoside reverse transcriptase inhibitors, and decreased susceptibility to enfuvirtide (28 fold-change). A co-receptor tropism test from 2013 revealed dual/mixed tropic virus.
Table 1.
Genotypic and Phenotypic Resistance Testing Reports
| Genotypea Resistance Mutations | Antiretroviral Drugs | Phenotypeb Fold Change | Phenotype Report Interpretation | |
|---|---|---|---|---|
| N(t)RTI | M41L, E44D, L74V, V118I, M184V, L210W, T215Y | Tenofovirc | 2.84 | Partially sensitive |
| Abacavir | 12 | |||
| Didanosine | 2.76 | |||
| Emtricitabinec | >MAX | Resistant | ||
| Lamivudine | >MAX | |||
| Stavudine | 3.75 | |||
| Zidovudine | 168 | |||
| NNRTI | A98G, K101H, V108I, V179F, Y181C, G190A, H221Y, K238R | Efavirenz | >MAX | Resistant |
| Etravirine | 117 | |||
| Nevirapine | >MAX | |||
| Rilpivirine | 30 | |||
| PI | L10I, V11L, I13V, V32I, L33F, E34D, M36L, M46I, I47V, I54M, Q58E, D60E, A71V, I84V, L89I, L90M | Darunavirc | 289 | Resistant |
| Atazanavir | 62 | |||
| Fosamprenavir | >MAX | |||
| Indinavir | 36 | |||
| Lopinavir | >MAX | Resistant | ||
| Nelfinavir | 51 | |||
| Ritonavir | >MAX | |||
| Saquinavir | 15 | |||
| Tipranavir | >MAX | |||
| INSTI | V151I, N155H, E157Q | Dolutegravirc | 2.02 | Sensitive |
| Elvitegravir | 132 | Resistant | ||
| Raltegravir | 73 | |||
| CCR5 Inhibitors | N/A | Maraviroc | Dual/Mixed Tropic HIV | No activity anticipated |
| Entry Inhibitors | N/A | Enfuvirtide | 28 | Reduced susceptibility |
TRUGENE® HIV-1 Genotyping Test - sample from April 22, 2014
PhenoSense GT Plus Integrase, Monogram Biosciences, South San Francisco, CA - sample from May 19 2014
Bolded information represents the drugs prescribed as part of the salvage regimen in this patient
Abbreviations: INSTI = integrase strand transfer inhibitor, NNRTI = non-nucleoside reverse transcriptase inhibitor, N(t)RTI = nucleoside/nucleotide reverse transcriptase inhibitor, PI = protease inhibitor
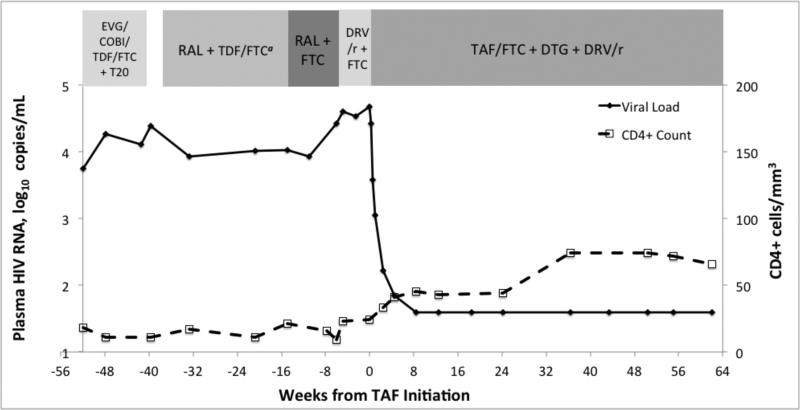
On 1 July, 2014, after discontinuation of TDF and while on a regimen of only raltegravir and emtricitabine, his SCr decreased to 1.49 mg/dL (132 μmol/L, eGFR 52 mL/min/1.73 m2) and his viral load increased by 0.57 log10 to 39,950 copies/mL (Figure 2), suggesting that TDF was responsible for partial viral suppression. In an attempt to prevent further accumulation of integrase mutations, raltegravir was discontinued and replaced with ritonavir-boosted darunavir. In August 2014, given his limited treatment options, we obtained co-formulated TAF 25mg/emtricitabine 200mg (Gilead Sciences, Foster City, CA) through a single patient investigational new drug (IND 123392) with approval from the FDA. A regimen of TAF 25 mg/emtricitabine 200 mg once daily, dolutegravir 50 mg twice daily, and ritonavir-boosted darunavir (100 mg/600 mg) twice daily was initiated. At that time, his CD4+ count was 24 cells/mm3, HIV RNA was 47,538 copies/mL, SCr was 1.30 mg/dL (115 μmol/L, eGFR 62 mL/min/1.73 m2), serum cystatin C was 1.37 mg/L (reference range 0.55 – 1.03 mg/L), urine albumin was 196 mg/L, and urine beta-2-microglobulin was >2.5 mg/L. After 2 weeks of the new ART regimen, his urine albumin was 182 mg/L, with a urine albumin to protein ratio (UAPR) of 19% and a urine albumin to creatinine ratio (UACR) of 122 mg/g. SMX/TMP was restarted at week 4. He achieved HIV RNA <40 copies/mL at week 8. At 15 months of follow-up, virological suppression was maintained, his CD4+ count increased to 66 cells/mm3, SCr remained stable at 1.41 mg/dL (125 μmol/L), eGFR was 55 mL/min/1.73 m2, cystatin C was 1.43 mg/L, urine beta-2-microglobulin remained at >2.5 mg/L, and urine albumin was 185 mg/L with a UAPR of 7% and a UACR of 95 mg/g. Dual-energy X-ray absorptiometry (DEXA) scans performed at 6 and 12 months of TAF therapy detected no change in bone mineral density (BMD) of hip and spine, compared to baseline
Figure 2.
Antiretroviral regimens, HIV viral load, and CD4+ cell count prior to and after initiating a salvage regimen with tenofovir alafenamide
COBI = cobicistat, DRV/r = ritonavir-boosted darunavir, DTG = dolutegravir, EVG = elvitegravir, FTC = emtricitabine, RAL = raltegravir, TAF = tenofovir alafenamide, TDF = tenofovir disoproxil fumarate, T20 = enfuvirtide
Discussion
Over 20 antiretroviral agents spanning six distinct mechanistic drug classes have been approved for clinical use. Clinical guidelines recommend managing treatment failure by including at least two, and preferably three, fully active agents in a salvage regimen (8). Two active agents, however, may not be available for patients with substantial treatment experience and mutations that confer cross-resistance to multiple antiretroviral drugs within a class.
Dolutegravir has not been shown to select for integrase inhibitor-resistant strains in treatment-naïve patients but is less active against strains with specific integrase mutations selected by raltegravir and elvitegravir (9, 10). In our patient, while awaiting approval of a single patient IND to use TAF, we discontinued raltegravir and initiated ritonavir-boosted darunavir to avoid emergence of additional integrase resistance mutations, particularly the mutation at position Q148, which has been associated with virologic failure on optimized regimens (7, 10).
Our patient's phenotypic testing reported sensitivity to dolutegravir, but only partial to no susceptibility to the remaining components of his antiretroviral regimen, including substantial increases in IC50 to TDF (2.84 fold, partial sensitivity from 1.4 to 4 fold-change), darunavir (289-fold) and emtricitabine (>max). The phenotype showed a lesser 15-fold change in susceptibility to saquinavir; however, we chose darunavir over saquinavir, as there is broad experience with darunavir in salvage therapy in clinical trials and in clinical practice (11). With dolutegravir and darunavir, higher doses and blood concentrations can increase antiretroviral activity against HIV strains with intermediate resistance to these drugs (12, 13). It is unknown if TFV exerts a similar dose-response effect. The history of AKI prohibited use of higher TDF doses; in contrast, TAF retains greater activity in vitro than TDF against partially sensitive HIV-1 strains on the basis of greater peripheral blood mononuclear cell (PBMC) TFV concentrations (1, 2, 14). Unlike TDF, after oral absorption, TAF is stable in plasma and mainly metabolized to the pharmacologically active TFV-diphosphate after reaching its site of action by intracellular uptake by CD4+ cells (15). Compared to TDF, TAF resulted in 90% lower serum concentration of TFV and 6-fold higher PBMC TFV concentration (16). While virologic potency of TAF in patients with TFV-associated resistance is yet to be defined in vivo, TAF 25 mg daily provided a significantly greater decrease in HIV RNA than TDF 300 mg (−1.46 versus −0.97 log10 copies/mL, p=0.024) after 10 days of monotherapy in antiretroviral-naïve subjects (17). Our patient, taking TAF with only one fully active agent in dolutegravir, achieved a 1.6 log10 decrease in HIV RNA after seven days, and a >3.1 log10 decrease to <40 copies/mL by week 8, and maintained suppression through 48 weeks.
Two 48-week phase 3 studies of treatment-naïve subjects have demonstrated virologic non-inferiority and a favorable safety profile of TAF compared to TDF (5). Similarly, two randomized phase 3 studies of virologically suppressed subjects without kidney disease showed that subjects who switched from TDF to TAF had a higher rate of virologic suppression (97% to 93%, P<0.001), less proteinuria, and improvement in BMD after 48 weeks compared to subjects who continued on a TDF regimen (6), though DEXA scan detected no change in BMD of the hip and spine in our patient after 12 months of TAF. In a prospective cohort switch study of 242 virologically suppressed patients with mild to moderate renal dysfunction (Cockcroft-Gault eGFR of 30 to 69 ml/min), subjects who switched from TDF to TAF did not experience any change in Cockcroft-Gault eGFR and showed significantly reduced proteinuria by urine protein to creatinine ratio, UACR, urine beta-2-microglobulin and urine retinol-binding protein after 48 weeks (18). There are no published data on the tolerability of TAF in patients with previous AKI secondary to TDF, and it is unknown whether switching from TDF to TAF results in improvement of TDF–induced renal tubulopathy and proteinuria in these patients. Our patient continued to have stable moderate renal dysfunction with proteinuria during 12 months of TAF therapy. His UPCR, UACR and urine beta-2-microglobulin level continued to indicate tubulopathy, although glomerular disease secondary to hypertension could be contributory. As creatinine tubular secretion can be inhibited by dolutegravir (19), leading to falsely low eGFR calculations, we also monitored cystatin C concentration as another measure of renal function. The utility of cystatin C as an independent marker of renal function in HIV-infected patients may be complicated, however, as it may be increased by HIV viremia and inflammation (20). Nonetheless, both eGFR and cystatin C remained stable throughout TAF and dolutegravir treatment in our patient, with no evidence of worsening renal function.
Current guidelines recommend discontinuing TDF in patients experiencing progressive GFR decline without other identifiable causes and avoiding TDF in patients with chronic kidney disease (21, 22). This may limit treatment options in some patients, particularly those with hepatitis B virus co-infection and those with extensively drug resistant HIV. We report here, in a single case, the ability to safely use TAF in a patient with TDF-associated kidney injury with proteinuria and severely impaired eGFR. Moreover, TAF, as part of a salvage regimen, was successful in achieving viral suppression in the setting of six-class antiretroviral drug resistance. The role of TAF in patients with TFV-associated resistance mutations and renal dysfunction warrants further study.
Acknowledgement
We would like to acknowledge Dr. Vasilios Pyrgos for referring the patient to our protocol, and the patient for his participation in the study. The research was supported in part by the National Institute of Allergy and Infectious Diseases and the NIDDK Intramural Research Program. This project has also been funded in part with federal funds from the National Cancer Institute, National Institutes of Health (NIH), under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Tenofovir alafenamide (25mg)/emtricitabine (200mg) combination tablet was provided by Gilead Sciences, Foster City, CA
References
- 1.Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773–80. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Moyle GJ, Stellbrink HJ, Compston J, Orkin C, Arribas JR, Domingo P, et al. 96-Week results of abacavir/lamivudine versus tenofovir/emtricitabine, plus efavirenz, in antiretroviral-naive, HIV-1-infected adults: ASSERT study. Antivir Ther. 2013;18(7):905–13. doi: 10.3851/IMP2667. [DOI] [PubMed] [Google Scholar]
- 3.Huang JS, Hughes MD, Riddler SA, Haubrich RH. Bone mineral density effects of randomized regimen and nucleoside reverse transcriptase inhibitor selection from ACTG A5142. HIV Clin Trials. 2013;14(5):224–34. doi: 10.1310/hct1405-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markowitz M, Zolopa A, Squires K, Ruane P, Coakley D, Kearney B, et al. Phase I/II study of the pharmacokinetics, safety and antiretroviral activity of tenofovir alafenamide, a new prodrug of the HIV reverse transcriptase inhibitor tenofovir, in HIV-infected adults. J Antimicrob Chemother. 2014;69(5):1362–9. doi: 10.1093/jac/dkt532. [DOI] [PubMed] [Google Scholar]
- 5.Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015 doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 6.Mills A, Arribas JR, Andrade-Villanueva J, DiPerri G, Van Lunzen J, Koenig E, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. The Lancet Infectious diseases. 2015 doi: 10.1016/S1473-3099(15)00348-5. [DOI] [PubMed] [Google Scholar]
- 7.Castagna A, Maggiolo F, Penco G, Wright D, Mills A, Grossberg R, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014;210(3):354–62. doi: 10.1093/infdis/jiu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panel on Antiretroviral Guidelines for Adults and Adolescents [July, 2015];Guidelines for the use on antiretroviral agents in HIV-1-infected adults and adolescents. [page H1-11.]. Available from: http://www.aidsinfo/nih/gov/contentfiles/adultandadolescentgl.pdf.
- 9.Wainberg M, Anstett K, Mesplede T, Quashie P, Han Y, Oliveira M. The R263K mutation in HIV integrase that is selected by dolutegravir may actually prevent clinically relevant resistance to this compound. J Int AIDS Soc. 2014;17(4 Suppl 3):19518. doi: 10.7448/IAS.17.4.19518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fourati S, Charpentier C, Amiel C, Morand-Joubert L, Reigadas S, Trabaud MA, et al. Cross-resistance to elvitegravir and dolutegravir in 502 patients failing on raltegravir: a French national study of raltegravir-experienced HIV-1-infected patients. J Antimicrob Chemother. 2015;70(5):1507–12. doi: 10.1093/jac/dku535. [DOI] [PubMed] [Google Scholar]
- 11.Deeks ED. Darunavir: a review of its use in the management of HIV-1 infection. Drugs. 2014;74(1):99–125. doi: 10.1007/s40265-013-0159-3. [DOI] [PubMed] [Google Scholar]
- 12.Katlama C, Esposito R, Gatell JM, Goffard JC, Grinsztejn B, Pozniak A, et al. Efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. AIDS. 2007;21(4):395–402. doi: 10.1097/QAD.0b013e328013d9d7. [DOI] [PubMed] [Google Scholar]
- 13.Eron JJ, Clotet B, Durant J, Katlama C, Kumar P, Lazzarin A, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207(5):740–8. doi: 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margot NA, Johnson A, Miller MD, Callebaut C. Characterization of HIV-1 Resistance to Tenofovir Alafenamide (TAF) In Vitro. Antimicrob Agents Chemother. 2015 doi: 10.1128/AAC.01151-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bam RA, Yant SR, Cihlar T. Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19(7):687–92. doi: 10.3851/IMP2770. [DOI] [PubMed] [Google Scholar]
- 16.Mills A, Crofoot G, Jr., McDonald C, Shalit P, Flamm JA, Gathe J, Jr., et al. Tenofovir Alafenamide Versus Tenofovir Disoproxil Fumarate in the First Protease Inhibitor-Based Single-Tablet Regimen for Initial HIV-1 Therapy: A Randomized Phase 2 Study. J Acquir Immune Defic Syndr. 2015;69(4):439–45. doi: 10.1097/QAI.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 17.Ruane PJ, DeJesus E, Berger D, Markowitz M, Bredeek UF, Callebaut C, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63(4):449–55. doi: 10.1097/QAI.0b013e3182965d45. [DOI] [PubMed] [Google Scholar]
- 18.Pozniak A, Arribas JR, Gathe J, Gupta SK, Post FA, Bloch M, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48 week results from a single-arm, multi-center, open-label, Phase 3 study. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koteff J, Borland J, Chen S, Song I, Peppercorn A, Koshiba T, et al. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. British journal of clinical pharmacology. 2013;75(4):990–6. doi: 10.1111/j.1365-2125.2012.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhasin B, Lau B, Atta MG, Fine DM, Estrella MM, Schwartz GJ, et al. HIV viremia and T-cell activation differentially affect the performance of glomerular filtration rate equations based on creatinine and cystatin C. PloS one. 2013;8(12):e82028. doi: 10.1371/journal.pone.0082028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(9):e96–138. doi: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Society. EAC [November, 2015];European Guidelines for Treatment of HIV-Infected Adults Version 8.0. 2015 [page 44-46.]. Available from: http://www.eacsociety.org/files/2015_eacsguidelines_8.0-english_revised-20151104.pdf.