Fig. 2.
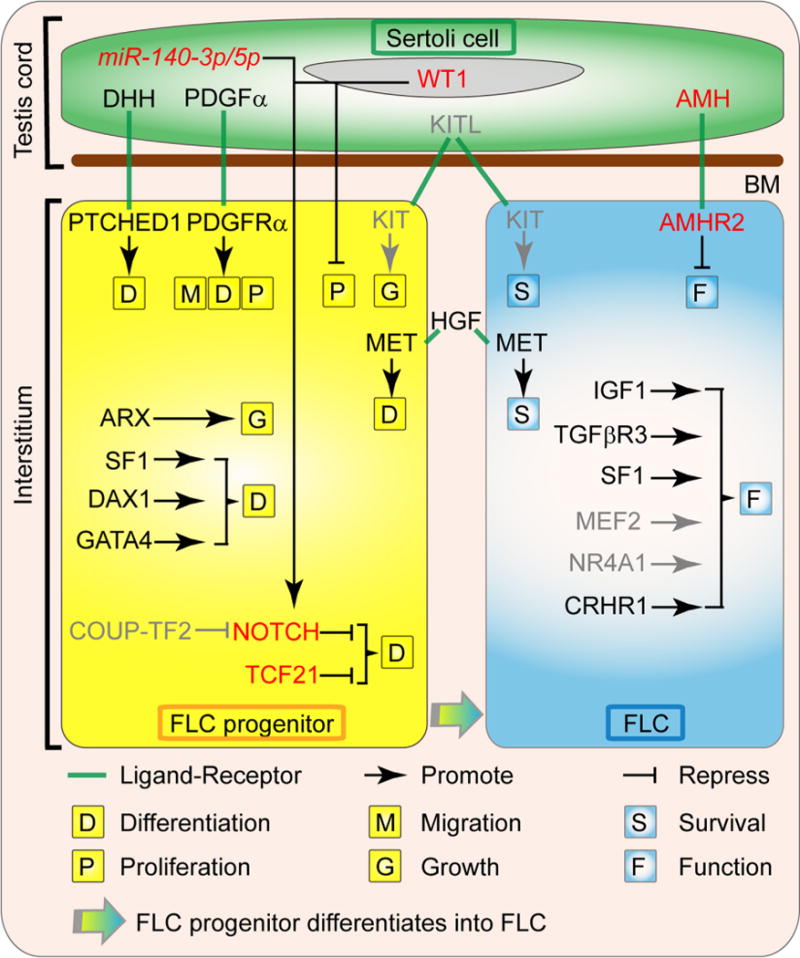
Factors involved in the ontogeny of fetal Leydig cells. The differentiation and steroidogenesis of FLC are regulated by diverse factors derived from Sertoli cells, FLC progenitors, FLCs and other interstitial components. The DHH (Desert hedgehog) and PDGFα (Platelet-derived growth factor A) are proteins secreted by the fetal Sertoli cells that are necessary for FLC ontogeny by inducing the commitment of the FLC progenitors through their receptor PTCH1 (Patched1) and PDGFRα (Platelet-derived growth factor receptor A), respectively. PDGFα also induces the migration and proliferation of FLC progenitors. The KITL (KIT ligand)/KIT (KIT oncogene) signaling is likely to be involved in FLC precursor growth and FLC survival. The HGF (Hepatocyte growth factor), which is localized in the connective tissue of the interstitial compartment, promotes FLC terminal development and protect FLCs from apoptosis. The transcription factor ARX (Aristaless-related homeobox) is essential for the establishment of FLC progenitors. SF1 (Steroidogenic factor 1, also called NR5A1 or AD4BP), DAX1 (dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1, also known as NR0B1 or AHCH) and GATA4 (GATA binding protein 4) also promote the differentiation of FLCs, while TCF21 (transcription factor 21, also known as POD1) and NOTCH signal pathway maintain the FLC progenitor pool. Sertoli cell transcription factor WT1 (Wilms’ tumor 1) and mircoRNA miR-140-3p/5p repress FLC differentiation via activating NOTCH signal pathway, while COUP-TF2 (Chicken Ovalbumin Upstream Promoter-Transcription Factor 2, also known as NR2F2) likely promotes FLC differentiation by repressing NOTCH signal pathway. After the establishment of FLC population during fetal testis development, IGF1 (insulin-like growth factor 1), TGFβR3 (Transforming growth factor, beta receptor III, also called betaglycan), SF1, MEF2 (Myocyte enhancer factor 2), NR4A1 (nuclear receptor subfamily 4, group A, member 1, also known as Nur77, NGFI-B, and TR3) and CRHR1 (Corticotropin-releasing hormone receptor 1) regulate the function of FLC, while Sertoli cell factor AMH (anti-Müllerian hormone) is a negative regulator of FLC function. Gray color indicates the regulations are inferred from ALCs. Abbreviations used: FLC, fetal Leydig cell; BM, basal membrane; DHH, Desert hedgehog; PTCH1, Patched1; PDGFα, Platelet-derived growth factor A; PDGFRα, Platelet-derived growth factor receptor A; KITL, KIT ligand; KIT, KIT oncogene; HGF, Hepatocyte growth factor; MET, met proto-oncogene; ARX, Aristaless-related homeobox; SF1, Steroidogenic factor 1; DAX1, dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1; GATA4, GATA binding protein 4; TCF21, transcription factor 21; WT1, Wilms’ tumor 1; COUP-TF2, Chicken Ovalbumin Upstream Promoter-Transcription Factor 2; IGF1, insulin-like growth factor 1; TGFβR3, Transforming growth factor, beta receptor III; MEF2, Myocyte enhancer factor 2; NR4A1, nuclear receptor subfamily 4, group A, member 1; CRHR1, Corticotropin-releasing hormone receptor 1; AMH, anti-Müllerian hormone; AMHR2, anti-Mullerian hormone type 2 receptor; miR-140-3p/5p, mircoRNA miR-140-3p/5p.
