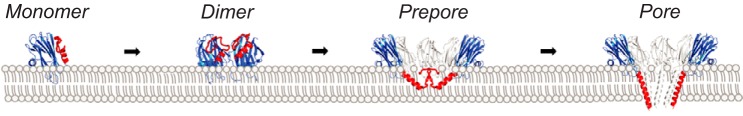
FIGURE 8.
Model for pore formation by FraC. A toxin monomer binds the membrane. The membrane promotes protein-protein interactions between monomers to produce a dimer (15) leading to prepore upon successive addition of monomer and/or dimers to the growing oligomer. In the prepore, the N-terminal α-helices are partially embedded in the membrane with their N termini exposed to the aqueous phase. The conversion to the transmembrane pore would be achieved by the concerted penetration and elongation of the helices across the lipid bilayer. The structures of the monomer, dimer, and pore were retrieved from the Protein Data Bank codes 3VWI, 4TSL, and 4TSY, respectively. The prepore illustrates a tentative model consistent with the presented in this study.