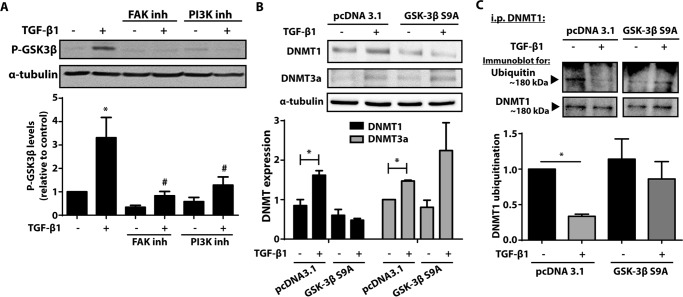
FIGURE 7.
TGF-β1 phosphorylated and inactivated GSK-3β, inhibiting DNMT1 ubiquitination. A, cells treated with 10 μm of the FAK inhibitor PF62271 or 10 μm of the PI3K inhibitor LY294002 in the presence or absence of TGF-β1 (2 ng/ml) were assayed by immunoblot for phosphorylation of GSK-3β at serine 9. The mean densitometric values of phosphorylated GSK-3β from seven independent experiments are shown below a representative immunoblot. *, p < 0.05 relative to untreated control; #, p < 0.05 relative to TGF-β1 treatment without inhibitors. B, CCL210 cells transfected with either control plasmid (pcDNA 3.1) or plasmid that overexpressed a constitutively active form of GSK-3β S9A were treated with or without TGF-β1 (2 ng/ml), and expression of DNMT1 and DNMT3a was assayed by immunoblot. The mean densitometric values relative to untreated pcDNA 3.1 control from three independent experiments are shown below the representative immunoblots. *, p < 0.05. C, cells transfected with either control or GSK-3β S9A plasmid were treated with MG-132 (1 μm) in the presence or absence of TGF-β1 (2 ng/ml). Cell lysates were immunoprecipitated for DNMT1 and immunoblotted for ubiquitin and DNMT1 (n = 4). *, p < 0.05. i.p., immunoprecipitation; inh, inhibitor.