FIGURE 1.
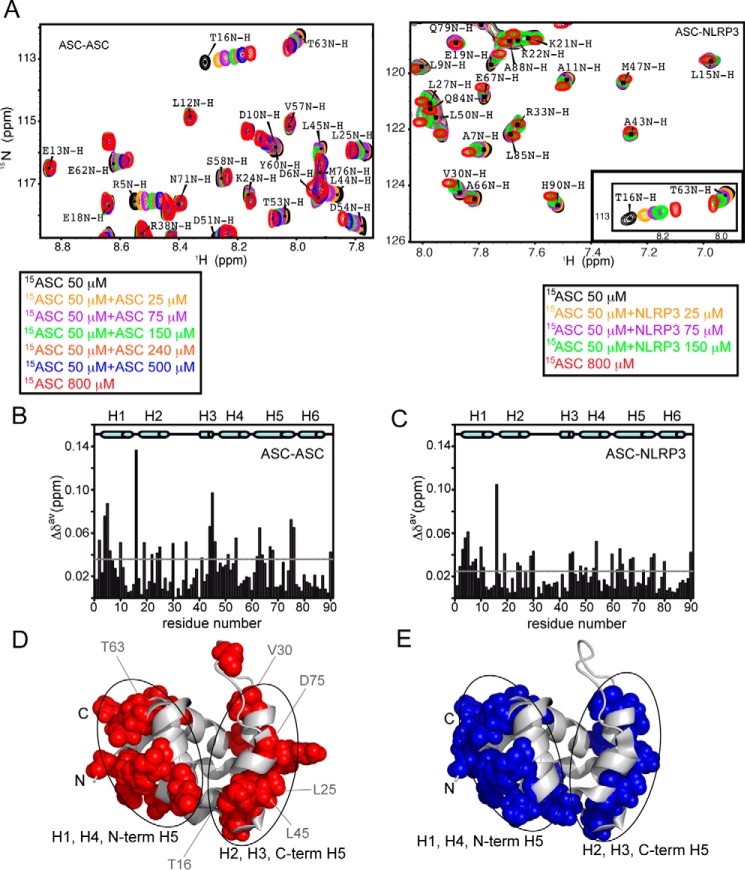
ASC PYD interacts with other PYDs through two equivalent interfaces. A, superimposed SOFAST-HMQC spectra resulting from the titration of 15N-labeled (constant concentration) ASC PYD with unlabeled ASC PYD (increasing concentration, left). Some signals (e.g. Leu-12 NH) remain unmodified, and other show clear changes in δ upon ASC·ASC binding (e.g. Arg-5, Thr-16, or Asp-54 NHs). Similar shifts were observed upon titration with unlabeled NLRP3 PYD (right). For comparison, the inset shows a representative region of the spectra in ASC·NLRP3 titrations where the observed shifts upon binding are equivalent to those found in ASC·ASC. B and C, chemical shift changes upon binding versus residue sequence for ASC·ASC (B) and ASC·NLRP3 (C) interactions. Dashed lines represent the threshold value calculated as 1.5 times the S.D. of the average chemical shift change obtained as explained under “Experimental Procedures.” D and E, mapping on ASC PYD structure of exposed residues with δ changes above the threshold value upon ligand binding for ASC·ASC (D, in red) and ASC·NLRP3 (E, in blue).