FIGURE 11.
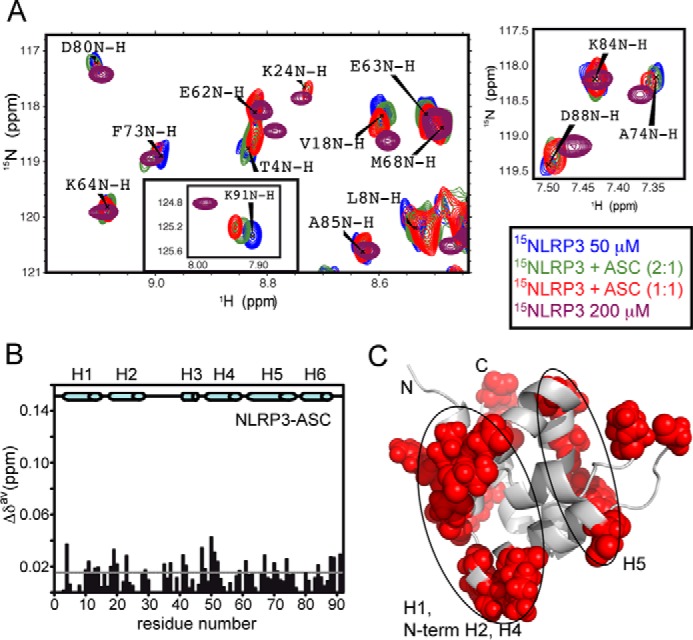
NLRP3 PYD interacts with ASC PYD through similar interfaces. A, superimposed SOFAST-HMQC spectra acquired in the titration of 15N-labeled NLRP3 with ASC. NLRP3 severe aggregation precluded titration data at molar ratios higher than 1:1. Peak shifts upon titration with ASC reproduce those observed at high NLRP3 concentration, indicating that NLRP3 PYD self-association and its interaction with ASC PYD employ identical interfaces. B and C, mapping of the NLRP3 residues undergoing above-threshold δ upon ASC binding shows similar interfaces (H1, N-terminal H2, H4, and H5) to those found in ASC (Fig. 1) and in pyrin (44). Note that the magnitude of δ in this case is significantly lower than for ASC PYD (Fig. 1, B and C; the scale on the y axis is maintained for comparison), and those residues whose resonances appeared in the central blurb were discarded from this mapping (Cys-6, Lys-7, Leu-8, Ala-9, Lys-21, Leu-27, Lys-34, Leu-55, Ile-57, Ala-65, Arg-78, Arg-79, and Tyr-82).
