FIGURE 2.
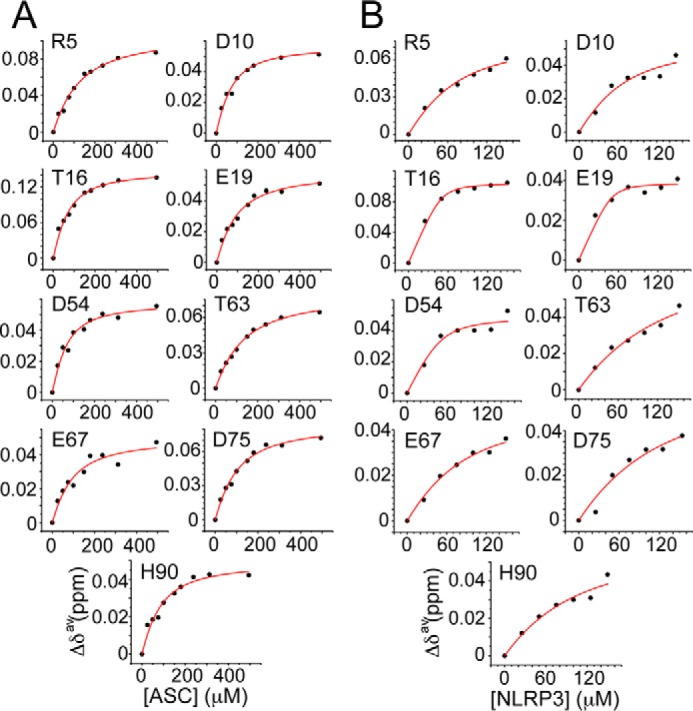
Dissociation constant (KD) values indicate preference of ASC PYD for NLRP3 versus self-association. Changes in chemical shift versus protein concentration for the ASC·ASC (A) and the ASC·NLRP3 (B) interaction. KD values for the ASC·ASC interaction are as follows: Arg-5 (98 ± 14 μm (r = 0.99)); Asp-10 (43 ± 8 μm (r = 0.98)); Thr-16 (41 ± 8 μm (r = 0.98)); Glu-19 (63 ± 12 μm (r = 0.98)); Asp-54 (38 ± 10 μm (r = 0.97)); Thr-63 (106 ± 11 μm (r = 0.99)); Glu-67 (65 ± 25 μm (r = 0.93)); Asp-75 (74 ± 11 μm (r = 0.99)); and His-90 (56 ± 15 μm (r = 0.96)). KD values for the ASC·NLRP3 interaction are as follows: Arg-5 (42 ± 17 μm (r = 0.98)); Asp-10 (37 ± 36 μm (r = 0.92)); Thr-16 (3 ± 1 μm (r = 0.99)); Glu-19 (2 ± 3 μm (r = 0.96)); Asp-54 (8 ± 7 μm (r = 0.95)); Thr-63 (101 ± 58 μm (r = 0.97)); Glu-67 (55 ± 20 μm (r = 0.99)); Asp-75 (95 ± 85 μm (r = 0.94)); and His-90 (71 ± 47 μm (r = 0.96)). Note that the fittings show poor quality for ASC·NLRP3 because of the lower ratio reached (1:3).
