FIGURE 4.
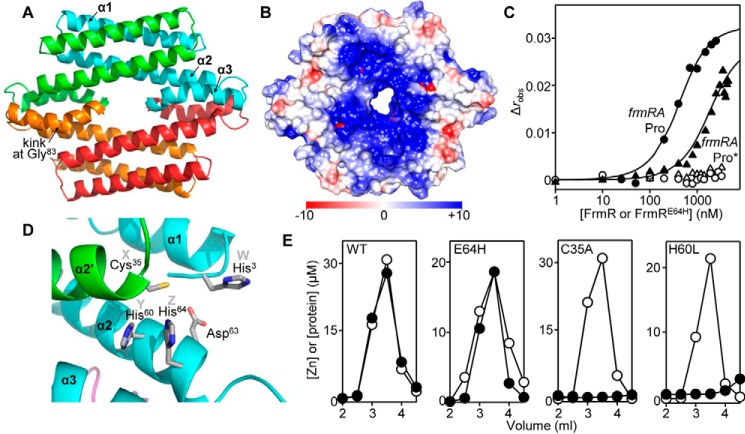
Structure of FrmRE64H and inferred Zn(II)/Co(II)-sensing site. A, ribbon representation of the 2.19 Å resolution crystal structure of FrmRE64H tetramer (Protein Data Bank code 5LCY; see Table 2 for a summary of the crystallographic data). Each monomer is colored differently, and secondary structural units are labeled on the cyan monomer. B, electrostatic surface potential of FrmRE64H tetramer using Chimera (103). The color scale is from −10 (negative potential; red) to +10 (positive potential; blue) kcal/mol·e. C, anisotropy change upon titration of a limiting concentration (10 nm) of frmRAPro (solid symbols) or frmRAPro* (half-site defined in Fig. 2D; open symbols) with FrmR (circles) or FrmRE64H (triangles) in the presence of 5 mm EDTA. The lines are fits of the data to a model describing a 2:1 protein tetramer (nondissociable)/DNA stoichiometry (binding with equal affinity) (50, 86). D, expansion of the dimeric interface with backbone helices from two different monomers shaded green and cyan (the same colors as used in A). The inferred Zn(II)/Co(II)-binding site comprises Cys35 from α2′, and His60 and His64 from α2 (belonging to the XYZ motif required for metal binding in DUF156 members CsoR, RcnR, and InrS (39, 46, 68), with His3 from α1 (position W (46, 61)) and Asp63 presenting candidate fourth ligands. E, analysis of fractions (0.5 ml) for protein by Bradford assay (open circles) and metal by inductively coupled plasma MS (filled circles) following size exclusion chromatography of FrmR, FrmRE64H, FrmRC35A (50 μm, monomer), or FrmRH60L (in this case, [monomer] = 32.5 μm), preincubated with 150 μm ZnCl2.