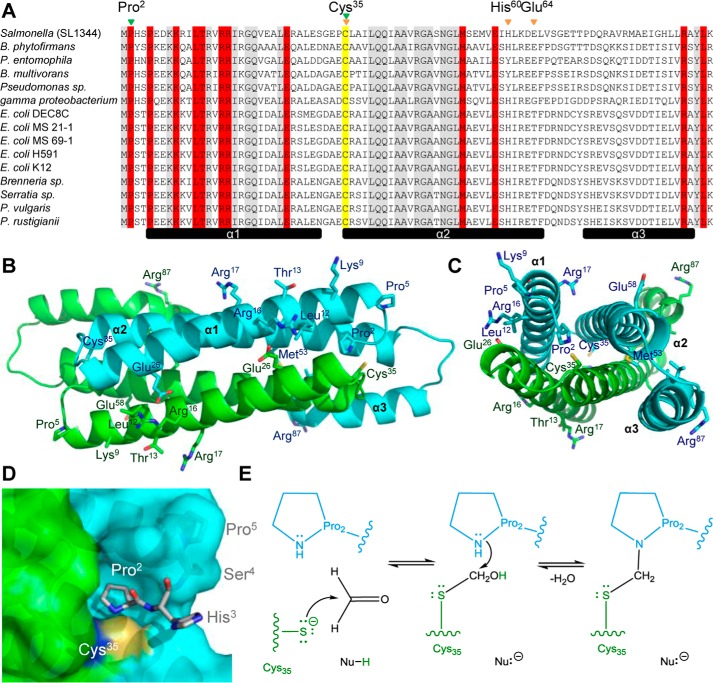
FIGURE 5.
Conservation of residues in the DUF156 FrmR subgroup and proposed formaldehyde-sensing site. A, alignment of Salmonella FrmR with nonredundant UniProtKB DUF156 sequences previously attributed to the FrmR subgroup (45). Organism details and UniProtKB identifiers are outlined under “Experimental Procedures.” Highlighted in gray are residues conserved in both FrmR and RcnR subgroups. Highlighted in red are residues conserved in the FrmR but not RcnR subgroup. Highlighted in yellow is the invariant cysteine present in all DUF156 proteins. The secondary structure elements of the FrmRE64H crystal structure are shown below (black bars). The inferred Zn(II)/Co(II)-sensing site is identified by orange arrows. The proposed formaldehyde sensing site is identified by green arrows. B and C, dimeric representation of FrmRE64H with the side chains for Cys35 and FrmR subgroup-specific residues labeled. Each monomer is colored differently (using the same colors as in Fig. 3A) with secondary structure units labeled on the cyan subunit. D, solvent-accessible surface representation of the proposed formaldehyde-binding site, which comprises Pro2 (subunit 1, cyan) and Cys35 (subunit 2, green). E, proposed reaction of formaldehyde with FrmR Cys35 (green) followed by Pro2 (cyan) (both deprotonated ultimately to water) via an S-hydroxymethyl intermediate. The reciprocal reaction with Pro2 followed by Cys35 via an N-methylol intermediate is also possible. In both cases, a methylene bridge (black) between the two residues is the final product. The nucleophile(s) responsible for deprotonation of Cys35 and Pro2 remain unknown.