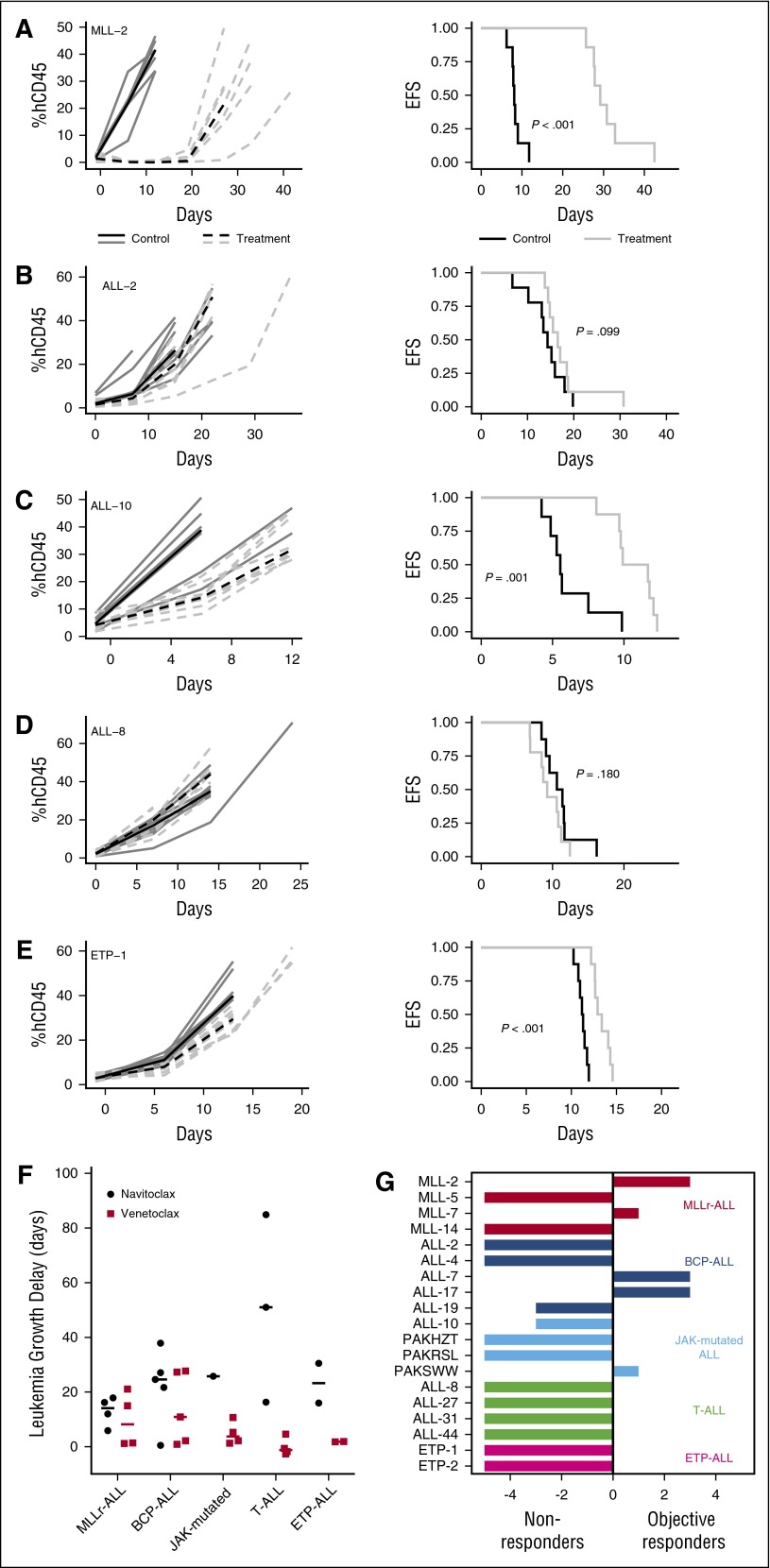
Figure 1.
In vivo single-agent venetoclax responses of pediatric ALL xenografts. Responses of representative xenografts from the (A) MLLr-ALL, (B) BCP-ALL, (C) JAK-mutated ALL, (D) T-ALL, and (E) ETP-ALL subpanels treated with venetoclax (100 mg/kg for 21 days, dotted lines) or vehicle control (solid lines); results from individual mice are represented by gray lines, whereas the black lines summarize the outcome for each cohort. In each case, the left panels represent the %huCD45+ of individual mice over time, whereas the right panels show the proportion of mice remaining event free. (F) Leukemia growth delay (T − C) of ALL xenograft subtypes following treatment with navitoclax or venetoclax. Each data point represents the median cohort leukemia growth delay for each xenograft; the horizontal bar represents the median for each ALL subtype. Data from the navitoclax cohort have been published previously27 and are included here for comparison. Statistical comparison between cohorts treated with navitoclax vs venetoclax was by unpaired Student t tests corrected for multiple comparisons using the Bonferroni method. (G) “COMPARE-like” plot of the difference between the median ORM of xenografts shown in Table 1 and the midpoint response (which corresponds to a score of 5). Bars to the right or left of the midpoint represent objective responses or nonobjective responses, respectively. Xenografts achieving a PR (median ORM 6), CR (median ORM 8), or MCR (median ORM 10) classify as responders, whereas those with progressive disease (PD1, median ORM 0; or PD2, median ORM 2) classify as nonresponders.