Abstract
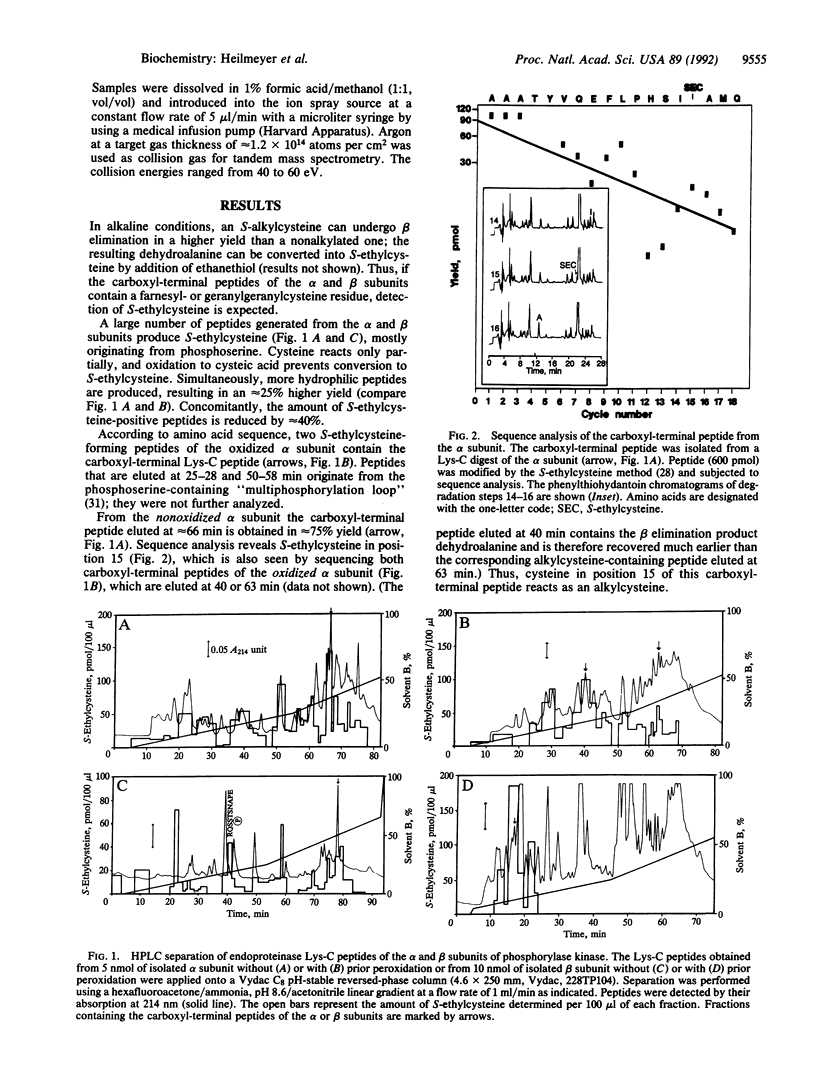
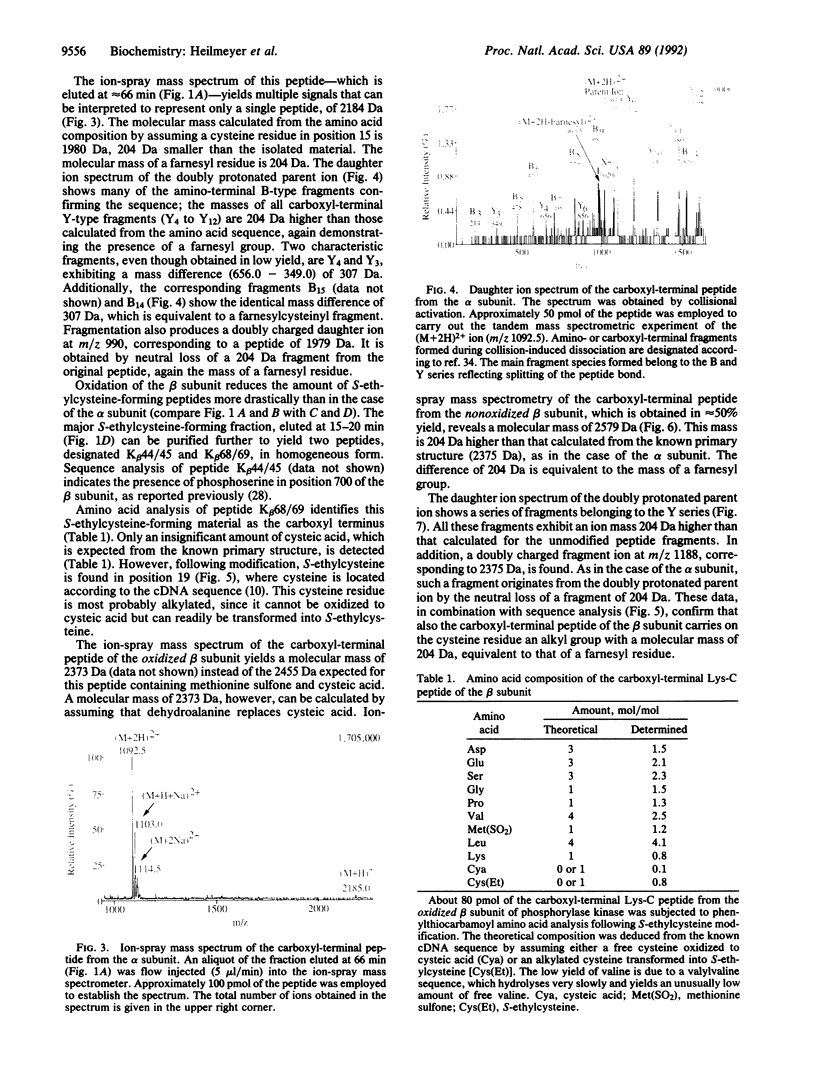
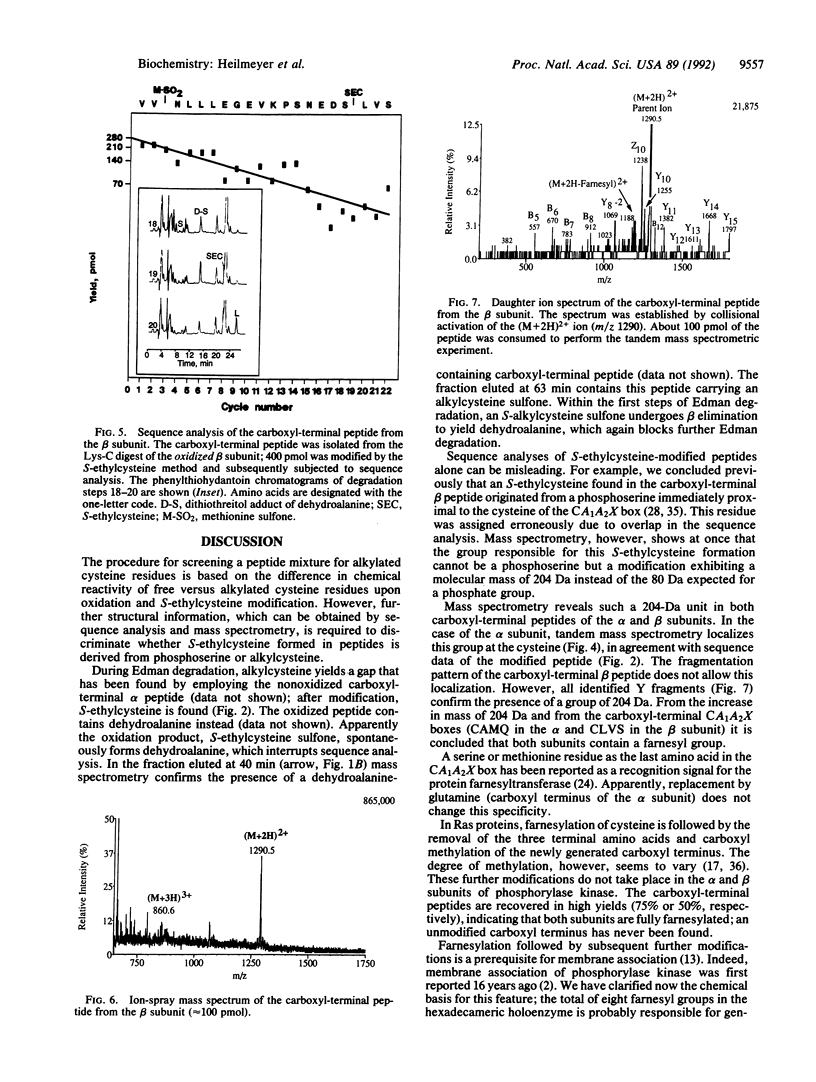
The primary structure of the alpha and beta subunits of phosphorylase kinase reveals that both proteins contain a carboxyl-terminal CA1A2X motif (where C is cysteine, A1 and A2 are aliphatic amino acids, and X is an uncharged amino acid), the recognition signal for a protein polyisoprenyltransferase. The product, a polyisoprenylated cysteine, can be detected by phenylthiocarbamoylamino acid analysis and by microsequencing following conversion to S-ethylcysteine. Mass spectrometry confirms a covalently linked farnesyl residue in both subunits. Tandem mass spectrometry localizes these modifications at the cysteine residues present in the carboxyl-terminal CAMQ and CLVS sequences of the alpha and beta subunits, respectively. Membrane association of phosphorylase kinase, probably mediated by these farnesyl residues, is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988 Dec 5;263(34):18236–18240. [PubMed] [Google Scholar]
- Biemann K. Appendix 5. Nomenclature for peptide fragment ions (positive ions). Methods Enzymol. 1990;193:886–887. doi: 10.1016/0076-6879(90)93460-3. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb J. W., Heilmeyer L. M., Jr High performance liquid chromatography purification and structural characterization of the subunits of rabbit muscle phosphorylase kinase. J Biol Chem. 1984 May 25;259(10):6346–6350. [PubMed] [Google Scholar]
- Dombradi V. K., Silberman S. R., Lee E. Y., Caswell A. H., Brandt N. R. The association of phosphorylase kinase with rabbit muscle T-tubules. Arch Biochem Biophys. 1984 May 1;230(2):615–630. doi: 10.1016/0003-9861(84)90443-0. [DOI] [PubMed] [Google Scholar]
- Epstein W. W., Lever D. C., Rilling H. C. Prenylated proteins: synthesis of geranylgeranylcysteine and identification of this thioether amino acid as a component of proteins in CHO cells. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7352–7354. doi: 10.1073/pnas.87.19.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold A. A., Johnson D. I., Farnsworth C. C., Gelb M. H., Judd S. R., Glomset J. A., Tamanoi F. Protein geranylgeranyltransferase of Saccharomyces cerevisiae is specific for Cys-Xaa-Xaa-Leu motif proteins and requires the CDC43 gene product but not the DPR1 gene product. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4448–4452. doi: 10.1073/pnas.88.10.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y., Takao T., Ohguro H., Yoshizawa T., Akino T., Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990 Aug 16;346(6285):658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- Georgoussi Z., Heilmeyer L. M., Jr Evidence that phosphorylase kinase exhibits phosphatidylinositol kinase activity. Biochemistry. 1986 Jul 1;25(13):3867–3874. doi: 10.1021/bi00361a019. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Gelb M. H., Farnsworth C. C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990 Apr;15(4):139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Gröschel-Stewart U., Jennissen H. P., Heilmeyer L. M., Jr, Varsani M. Localization of Ca2+-dependent protein kinases in various tissues by the immunofluorescent technique. Int J Pept Protein Res. 1978 Sep;12(3):177–180. doi: 10.1111/j.1399-3011.1978.tb02883.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I., Marshall C. J., Hancock J. F. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 1989 Apr;8(4):1093–1098. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. W., Thieleczek R., Varsányi M., Heilmeyer L. M., Jr Compartmentalized ATP synthesis in skeletal muscle triads. Biochemistry. 1992 Jan 21;31(2):377–384. doi: 10.1021/bi00117a010. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hessová Z., Thieleczek R., Varsányi M., Falkenberg F. W., Heilmeyer L. M., Jr Monoclonal antibodies to rabbit skeletal muscle phosphorylase kinase. Probes for studies of subunit function. J Biol Chem. 1985 Aug 25;260(18):10111–10117. [PubMed] [Google Scholar]
- Hörl W. H., Heilmeyer L. M., Jr Evidence for the participation of a Ca2+-dependent protein kinase and protein phosphatase in the regulation of the Ca2+ transport ATPase of the sarcoplasmic reticulum. 2. Effect of phosphorylase kinase and phosphorylase phosphatase. Biochemistry. 1978 Mar 7;17(5):766–772. doi: 10.1021/bi00598a002. [DOI] [PubMed] [Google Scholar]
- Jennissen H. P., Lahr P. Calcium-dependent adsorption and desorption of phosphorylase kinase on membrane fractions of sarcoplasmic reticulum. FEBS Lett. 1980 Nov 17;121(1):143–148. doi: 10.1016/0014-5793(80)81284-1. [DOI] [PubMed] [Google Scholar]
- Kamiya Y., Sakurai A., Tamura S., Takahashi N. Structure of rhodotorucine A, a novel lipopeptide, inducing mating tube formation in Rhodosporidium toruloides. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1077–1083. doi: 10.1016/0006-291x(78)91505-x. [DOI] [PubMed] [Google Scholar]
- Kilimann M. W., Zander N. F., Kuhn C. C., Crabb J. W., Meyer H. E., Heilmeyer L. M., Jr The alpha and beta subunits of phosphorylase kinase are homologous: cDNA cloning and primary structure of the beta subunit. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9381–9385. doi: 10.1073/pnas.85.24.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 1990 Dec;4(15):3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- Mayer S. E., Krebs E. G. Studies on the phosphorylation and activation of skeletal muscle phosphorylase and phosphorylase kinase in vivo. J Biol Chem. 1970 Jun;245(12):3153–3160. [PubMed] [Google Scholar]
- Meyer F., Heilmeyer L. M., Jr, Haschke R. H., Fischer E. H. Control of phosphorylase activity in a muscle glycogen particle. I. Isolation and characterization of the protein-glycogen complex. J Biol Chem. 1970 Dec 25;245(24):6642–6648. [PubMed] [Google Scholar]
- Meyer H. E., Hoffmann-Posorske E., Heilmeyer L. M., Jr Determination and location of phosphoserine in proteins and peptides by conversion to S-ethylcysteine. Methods Enzymol. 1991;201:169–185. doi: 10.1016/0076-6879(91)01016-u. [DOI] [PubMed] [Google Scholar]
- Meyer H. E., Meyer G. F., Dirks H., Heilmeyer L. M., Jr Localization of phosphoserine residues in the alpha subunit of rabbit skeletal muscle phosphorylase kinase. Eur J Biochem. 1990 Mar 10;188(2):367–376. doi: 10.1111/j.1432-1033.1990.tb15413.x. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Casey P. J., Gilman A. G., Gutowski S., Sternweis P. C. G protein gamma subunits contain a 20-carbon isoprenoid. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss Y., Stradley S. J., Gierasch L. M., Brown M. S., Goldstein J. L. Sequence requirement for peptide recognition by rat brain p21ras protein farnesyltransferase. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):732–736. doi: 10.1073/pnas.88.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra M. C., Reiss Y., Casey P. J., Brown M. S., Goldstein J. L. Protein farnesyltransferase and geranylgeranyltransferase share a common alpha subunit. Cell. 1991 May 3;65(3):429–434. doi: 10.1016/0092-8674(91)90460-g. [DOI] [PubMed] [Google Scholar]
- Thieleczek R., Behle G., Messer A., Varsanyi M., Heilmeyer L. M., Jr, Drenckhahn D. Localization of phosphorylase kinase subunits at the sarcoplasmic reticulum of rabbit skeletal muscle by monoclonal and polyclonal antibodies. Eur J Cell Biol. 1987 Oct;44(2):333–340. [PubMed] [Google Scholar]
- Varsanyi M., Tölle H. G., Heilmeyer M. G., Jr, Dawson R. M., Irvine R. F. Activation of sarcoplasmic reticular Ca2+ transport ATPase by phosphorylation of an associated phosphatidylinositol. EMBO J. 1983;2(9):1543–1548. doi: 10.1002/j.1460-2075.1983.tb01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorburger K., Kitten G. T., Nigg E. A. Modification of nuclear lamin proteins by a mevalonic acid derivative occurs in reticulocyte lysates and requires the cysteine residue of the C-terminal CXXM motif. EMBO J. 1989 Dec 20;8(13):4007–4013. doi: 10.1002/j.1460-2075.1989.tb08583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Howald W., Fung B. K., Clarke S., Gelb M. H., Glomset J. A. Brain G protein gamma subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Goodwin G. W., Ghomashchi F., Glomset J. A., Gelb M. H. A protein geranylgeranyltransferase from bovine brain: implications for protein prenylation specificity. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5302–5306. doi: 10.1073/pnas.88.12.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander N. F., Meyer H. E., Hoffmann-Posorske E., Crabb J. W., Heilmeyer L. M., Jr, Kilimann M. W. cDNA cloning and complete primary structure of skeletal muscle phosphorylase kinase (alpha subunit). Proc Natl Acad Sci U S A. 1988 May;85(9):2929–2933. doi: 10.1073/pnas.85.9.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]