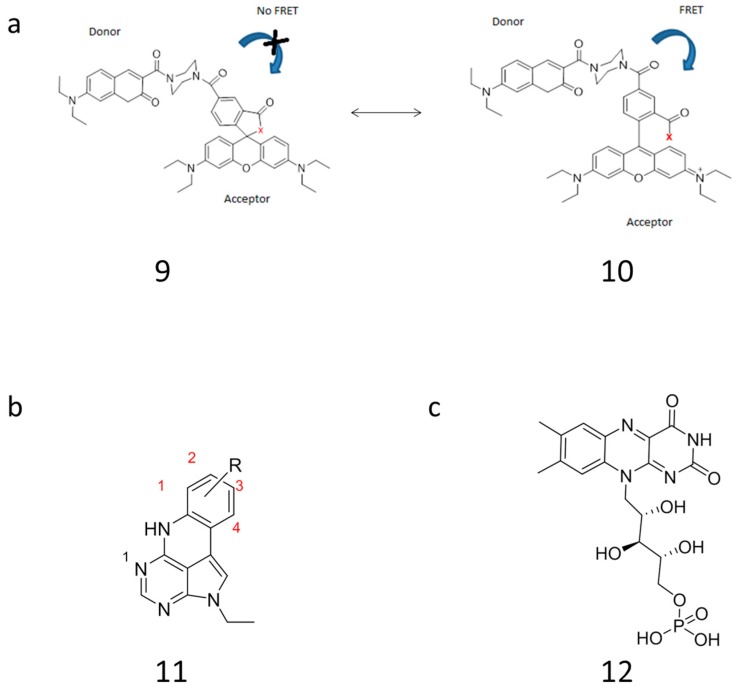
Figure 6.
Computational design of new FRET dyes: (a) Rhodamine undergoes a very characteristic absorption change between the ring opening and the ring-closing conformation. Yuan et al. improve this mechanism for imagining of small molecular targets such as Cu2+, NO, HOCl by separation of the interaction site (denoted x) and the energy donor [103]; (b) Quadracyclic adenine analogues with the different substituents, R, was introduced by Larsen et al. Adenine analogues substituted at position 1 and 2 with cyanogroups showed a stable fluorescence quantum yield and environment-sensitive emission. Both properties make them suitable for monitoring nucleic acids systems [104]. R: fluorine-, methoxy- and cyanogroups; (c) Molecular structure of flavin mononucleotide (FMN) [105].