Abstract
As part of an international effort to develop vaccines for Theileria lestoquardi, we undertook a limited screen to test T. lestoquardi orthologues of antigens recognised by CD8+ T lymphocyte responses against T. annulata and T. parva in cattle. Five MHC defined sheep were immunized by live T. lestoquardi infection and their CD8+ T lymphocyte responses determined. Thirteen T. lestoquardi orthologues of T. parva and T. annulata genes, previously shown to be targets of CD8+ T lymphocyte responses of immune cattle, were expressed in autologous fibroblasts and screened for T cell recognition using an IFNγ assay. Genes encoding T. lestoquardi antigens Tl8 (putative cysteine proteinase, 349 aa) or Tl9 (hypothetical secreted protein, 293 aa) were recognise by T cells from one animal that displayed a unique MHC class I genotype. Antigenic 9-mer peptide epitopes of Tl8 and Tl9 were identified through peptide scans using CD8+ T cells from the responding animal. These experiments identify the first T. lestoquardi antigens recognised by CD8+ T cell responses linked to specific MHC class I alleles.
Introduction
Theileria species are tick-transmitted hemoprotozoan parasites infecting wild and domestic ungulates in many areas of the world. The most economically important species are T. parva and T. annulata, which are pathogenic to cattle, and T. lestoquardi (formerly T. hirci), which is pathogenic to sheep. Sheep are valuable commodities in North Africa, Asia, and the Middle East, and there is a need for better prevention and/or treatment measures in order to reduce the economic burden of disease caused by T. lestoquardi.
Infection with T. lestoquardi causes an acute disease known as malignant ovine theileriosis. Clinical signs include loss of condition, coughing, lethargy, enlargement of lymph nodes, and fever [1]. Mortality levels of up to 73% have been reported for malignant ovine theileriosis [2] and the disease can lead to reduced productivity and spontaneous abortions in survivors [3]. T. lestoquardi is transmitted by Hyalomma anatolicum ticks [4] and possibly other Hyalomma spp. [5, 6] and, as with most vector-borne diseases, control of transmission is challenging [7]. In addition, current disease control methods are limited to treatment with a theilericidal compound, buparvaquone [8, 9], and in some countries, vaccination with attenuated parasitized cell lines [10–12]. These methods are not easy to apply successfully. Preparation of attenuated T. lestoquardi- infected cell line vaccines suitable for vaccination requires prolonged in vitro passage and thorough testing in vivo, and distribution of the vaccine is dependent on a cold chain. There are also issues with quality control, reproducibility and potential reversion to virulence [13, 14]. Furthermore, the mechanisms of attenuation are only partially understood [14–16]. A subunit vaccine would circumvent the logistical constraints of attenuated cell line vaccines. However, development of a subunit vaccine requires a greater understanding of protective immune responses against T. lestoquardi and the antigens that they recognise.
Studies of immune responses to, and antigen identification in, T. lestoquardi are lagging behind those for T. parva and T. annulata for which antigens were identified through screening of random T. parva and selected T. annulata and T. parva schizont cDNA clones with potential to transform host cells [17–19]. Although the disease produced by these parasites and the immune responses they induce are very similar, evidence of genetic and antigenic similarities are most evident for T. lestoquardi and T. annulata. These include cross-reactivity of T. lestoquardi antisera with T. annulata antigens [20], serological identification of T. lestoquardi proteins with amino acid sequence similarity to T. annulata and T. parva [21, 22], infection of similar cell types by T. lestoquardi and T. annulata in sheep and cattle respectively [23], and the higher similarity between T. lestoquardi and T. annulata 18S rRNA sequences [24]. There have been no reports on cellular responses to antigens conserved across T. lestoquardi, T. annulata and T. parva; but these may occur given the identification of a conserved T cell antigen between T. annulata and T. parva [19].
There is evidence that immunity to T. parva and T. annulata in cattle involves T cell mediated responses; both CD4+ and CD8+ T cells recognise parasitized leukocytes [25, 26]. An important role for CD8+ T cell responses in protection against T. parva has been demonstrated by adoptive transfer of immune CD8+ T cells [27], and it has been proposed that help from CD4+ T cells may also be required [28]. CD8+ T cell responses to T. parva and T. annulata antigens are MHC class I restricted [29, 30], and in individual animals the responses are frequently focused on a few immunodominant antigens, which differ depending on the MHC genotype of the animal [19, 31].
Based on genetic and pathogenic similarities of T. lestoquardi to T. annulata and T. parva, we propose that similar responses are likely to be involved in immunity against T. lestoquardi and that recognition of immunodominant T cell antigens orthologous to those of T. annulata/T. parva may occur. This study, therefore, aimed to obtain evidence of induction of CD8+ T cell responses by T. lestoquardi and to identify parasite antigens recognised by the specific CD8+ T cell response based on screening orthologues of those recognised in T. annulata and T. parva.
Methods
Ethics statement
Animal care and use were approved by the Royal Veterinary College Ethics Committee with the Home Office Project licence number PPL 60/3736. Animal work was carried out in accordance with the UK government Animals (Scientific Procedures) Act (ASPA) 1986.
Animals
Five adult Swaledale/Leicester cross sheep (approximately 4 years old) were used in this study. Animals were euthanized at the end of the study with lethal injection of barbituates.
Immunization
Sheep were immunized by subcutaneous administration of 1–3 × 106 cells of the T. lestoquardi-infected cell line, THS1 [32], as previously described [11]. Sheep were treated with buparvaquone (2.5mg kg-1, Bimeda, Ireland) if fever persisted for two consecutive days. One week after recovery from clinical reactions, sheep were re-challenged with the same number of autologous parasitized cells without treatment to confirm and boost their immunity. Health of the sheep was monitored by daily measurement of rectal temperature and palpation of the draining lymph node.
MHC class I and II genotyping
MHC class I genotyping was carried out as previously described [33] with some modifications. Total RNA was extracted with the Qiagen RNeasy Mini kit from cryopreserved lymphocytes of T. lestoquardi-infected sheep. Contaminating DNA was removed with the Turbo DNA-free™ kit (Applied Biosystems) before cDNA synthesis, which was carried out using the AMV LongAmp® Taq RT-PCR kit (New England Biolabs). Partial MHC class I sequences were amplified from cDNA samples using class I generic primers 416 (5' CGGCTACGTGGACGACAYG 3') and Cr (5' ATGGGTCACATGTGYCTTTG 3'), which bind within exons 1 and 3 [33], generating a 500 bp product. The cycling conditions for PCR were 94°C for 4 min, 30 cycles 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, with Promega Go Taq DNA polymerase. Amplicons were gel purified (QIAquick Gel Extraction kit, Qiagen) and Sanger sequenced (Eurofins UK) using the same primer set, resulting in multiple sequences of different alleles. Individual class I sequences were determined by cloning the purified amplicons into pGEM®-T Easy vector (Promega). Thirty transformants per sample were selected for bidirectional Sanger sequencing with vector-specific T7 and SP6 primers. Class I sequences were aligned using the SeqMan Pro programme within the DNASTAR Lasergene 11 package, and a consensus sequences of each allele was generated from a minimum of 3 independent clones. Each consensus allelic sequence was BLASTN searched against an in house database of known ovine class I sequences. Novel alleles were added to this database, and assigned a unique name that reflects the order in which they were identified. MHC class II genotyping was carried out as previously described [34]. The novel class II DRB1 sequence was validated by cloning the complete second exon sequence. The sequence was submitted to the ENA and IPD-MHC database for an official allelic nomenclature.
Generation of T. lestoquardi infected cell lines
T. lestoquardi-infected cell lines were obtained from peripheral blood or lymph nodes of infected sheep. Blood or lymph node aspirates taken from the draining lymph node nearest the site of challenge were collected daily in Alsever’s solution (Sigma Aldrich), from day 12 post-infection but before treatment with buparvaquone [35]. PBMC and lymph node mononuclear cells were separated from the blood or lymph node aspirate using Ficoll-Paque (GE Life Sciences) according to the manufacturer’s recommendations, and resuspended in culture medium (RPMI 1640 Glutamax medium, Life Technologies, Gibco, Paisley, UK) supplemented with 10% FCS (GIBCO), penicillin-streptomycin (5000 units ml-1 and 5 mg ml-1, respectively, Sigma-Aldrich, Dorset, UK) and 50 μM of 2-mercaptoethanol (Sigma Aldrich). PBMC and/or lymph node cells were counted and dispensed at 2.5 × 106–1 × 107 cells per well in 2 ml culture medium in 24 well plates and cultured until parasitized cell lines were established [35]. The cultures were incubated at 37°C in 5% CO2.
Cloning of candidate T. lestoquardi genes
Total RNA was extracted from T. lestoquardi-infected PBMC of the study sheep, using either the RNeasy Mini Kit (Qiagen) or Trizol (Invitrogen), and cDNA was prepared using either AMV First strand cDNA synthesis kit (New England Biolabs) or QuantiTect Reverse Transcription kit (Qiagen). PCR was carried out either with Phusion Mastermix (Thermo Scientific) or Pfu DNA polymerase (Promega), and T. lestoquardi gene-specific primers were used for amplification of full-length cDNA (S1 Table). An exception was antigen Tl12, which was amplified from genomic DNA of T. lestoquardi infected PBMC. Genomic DNA was extracted using the DNeasy Blood and Tissue kit (Qiagen). Amplicons were cloned into a modified pMax expression vector (Lonza), containing a C-terminal V5-tag, by restriction-ligation. Exceptions were Tl2, Tl13245 (orthologue of T. annulata TA13245), and Tl16020 (orthologue of T. annulata TA16020), which were first cloned into pJET1.2 (Thermo Scientific) for gene amplification and then cloned into pMax either by restriction-ligation, or by sequence and ligation-independent cloning (SLIC) [36] (S1 Table). Briefly, SLIC involved linearization of pMax either by digestion with EcoRV and ScaI (Fermentas), or by inverse PCR with primers pMax_EcoRV and pMax_XhoI (S1 Table). Insert amplicons, generated from primers containing gene and vector-specific sequences, were purified and mixed with linearized vector, 0.6–1.5 U T4 DNA polymerase (New England Biolabs), 1 × BSA, and 1 × NEB Buffer 2 for 2.5 min. Recombinant plasmids of Tl1, 2, 3, 5, 6, 7, 8, 9, 10, 12, 16, 13245, or 16020 were transformed in either DH5α or DH10B cells (NEB, UK) and selected on LB plates supplemented with kanamycin (50 μg ml-1). Recombinant plasmids were Sanger sequenced to validate insert identity.
Culture of sheep skin fibroblast
Skin biopsies of approximately 1 cm2 were taken from immunized sheep to establish fibroblast cell lines with matching MHC to effector cells from the same sheep. Skin biopsies were washed in culture medium (RPMI with 10% FCS, and penicillin (5000 units ml-1)-streptomycin (5 mg ml-1)), cut into small pieces, adhered onto culture dishes mechanically, and air-dried for 1 h. Skin was then covered with culture media and incubated at 37°C in 5% CO2 for 1–3 weeks, or until fibroblast grew onto plastic wells. Culture media was changed weekly. Fibroblast cell lines were passaged every 3–4 days or as they reached confluency.
Transfection of ovine fibroblasts with T. lestoquardi antigenic genes
Plasmids for transfections were prepared from a single batch of 25 ml bacterial cultures using the Qiagen Plasmid Midi kit. Autologous fibroblast cells were seeded in 96 well plates at 1 × 104 per well in 100 μl of culture medium and incubated overnight at 37°C in 5% CO2. Transfection was carried out either with Lipofectamine LTX & Plus Reagent (Invitrogen) or Fugene (Promega) at reagent (μl): DNA (μg) ratios of 5:1 and 3:1, respectively. Lipofectamine transfected fibroblasts at 24 h and 48 h were examined for V5 tag expression by immunofluorescence as described previously [37]. Briefly, fibroblasts were seeded at 5 × 104 per well in 1 ml culture medium on glass coverslips in 24 well plates and incubated overnight at 37°C in 5% CO2. Cells were transfected either for 24 h or 48 h, fixed with 4% paraformaldehyde (Sigma Aldrich) and permeabilized with 0.2% Triton-X (Sigma Aldrich) for 10 min each. Cells were then blocked with 10% FCS for 30 min before incubation with anti-V5 mouse antibody (diluted 1:500, Invitrogen) for 1 h, followed by incubation with an anti-mouse goat antibody AlexaFluor 488 (diluted 1:1500, Invitrogen). Cells were counter-stained with 1 μM DAPI (Life Technologies) for 10 min, coverslips were blotted dry and mounted onto glass slides for microscopy with DAKO fluorescence mounting medium (Agilent Technologies). Fluorescence microscopy was carried out using an Olympus BX60 at 1000 × magnification, using appropriate filter cubes and the CoolLED pE excitation system (Nikon, UK). Images were captured using the Image-Pro Plus 5.0 (MediaCybernetics).
Stimulation and enrichment of CD8+ T cells
CD8+ T cells were prepared as previously described with slight modifications [38]. PBMC (effectors) were collected from immunized sheep at 28 days post immunization. Gamma irradiated (100 Gy) autologous T. lestoquardi infected cell lines generated as described above were used as stimulators. Effectors and stimulators were co-cultured at an effector to stimulator ratio of 20:1 in 2 ml of culture medium in 24 well plates, and incubated at 37°C for one week in 5% CO2. Cells were then harvested and dead cells removed using Ficoll-Paque (GE Life Sciences). Viable effector cells were re-stimulated again at an effector to stimulator ratio of 10:1. After an additional week, cells were harvested, separated on Ficoll-Paque, and enriched for CD8 by complement fixation to remove CD4, γδ T cells and NK T cells. To do so, a cocktail of the following mouse-anti sheep or bovine antibodies, each at 8 μg ml-1, was used: anti-CD4+ T cells (MCA2213GA; AbD Serotec), anti-γδ T cells (MCA838G; AbD Serotec), anti-NK T cells (MCA5933GA; AbD Serotec). The cocktail was added to 5 × 107 ml-1 effector cell suspension in equal volumes, for a final concentration of 4 μg ml-1 per antibody. The mixture was incubated on ice for 30 min for opsonisation to take place. One part of rabbit serum was added to two parts of the cells/antibody mixture and incubated at 37°C for 45 min. Thereafter, cells were washed in 10 ml culture medium, rested at 37°C for 2–3 h, spun on Ficoll-Paque to remove debris and harvested cells plated at 5 × 103 per well in a 96 well plate. Cells were re-stimulated with 1 × 103 irradiated T. lestoquardi cells in the presence of 100 U ml-1 of recombinant human (rhu) IL-2 (Proleukin®, Novartis). The cultures were incubated at 37°C in 5% CO2 and used as effectors in an IFNγ production assay 14–21 days later.
Phenotyping of enriched T cells
The phenotype of effector T cell populations was determined by FACS analysis using antibodies described above, as well as mouse- antisheep or antibovine MHC class I (MCA2444GA; AbD Serotec), B-cell (MCA2443GA; AbD Serotec) and CD8 (MCA2216GA; AbD Serotec). Cells (2 × 105–1 × 106) were mixed with an equal volume of primary antibody (final concentration 1 μg ml-1), incubated at 4°C for 30 min, washed three times using PBS, and resuspended in 50 μl FACS medium (2% horse serum in PBS). FITC-labelled goat anti-mouse IgG (AbD Serotec) was used as secondary antibody. Cells were incubated at 4°C for 30 min, washed as before and resuspended in FACSFlow (Becton Dickinson Biosciences) for data acquisition and analysis using either a FACSCalibur (Becton Dickinson Biosciences) or a FACSAria (Becton Dickinson Biosciences).
Cytotoxicity assay
T. lestoquardi infected cell lines (targets) were labelled with Indium oxine (111In) (GE Healthcare UK) by incubating 50 μl (1 × 106) cells with 0.185 Mbq of 111In for 15 min at 37°C. Labelled target cells were washed six times in washing medium (RPMI 1640 Glutamax medium with 2% FCS) then resuspended in culture medium. Effector cells, either in stimulated whole PBMC or stimulated and enriched CD8+ T cells prepared with T. lestoquardi-infected cell lines as described above, were mixed with labelled target cells in duplicates in two-fold dilutions starting at 80:1 in a final volume of 150 μl. Positive controls consisted of labelled target cells lysed with 100 μl of 0.2% Tween20, negative controls were unlabelled target cells, and all controls were performed in triplicate. Cells were incubated for 4 h at 37°C, resuspended in the same volume of culture medium and centrifuged at 250 × g for 10 min. Seventy-five μl of supernatant from each sample was measured for radioactivity in a gamma counter (WALLAC 1470, Perkin Elmer). Percentage cytotoxicity was calculated as release of 111In according to 100 × (test release–medium release) / (Tween20 release–medium release).
IFNγ ELISA
Fibroblasts transfected with plasmid DNA or treated with peptides overnight were washed three times before addition of 2.5 × 105 effector cells per well in 200 μl culture medium, and incubated for 72 h at 37°C in 5% CO2. Cell supernatants were harvested and analysed by IFNγ ELISA, according manufacturer’s instruction (MABTECHTM); ELISA reactions were recorded on a SpectraMax M2 (Molecular Devices) plate reader. All assays included the following controls: culture medium only, fibroblast only, effectors only, effectors and stimulators, fibroblast and stimulators, fibroblast transfected with plasmid expressing GFP, and for peptide assays—fibroblasts transfected with plasmid expressing Tl8 or Tl9.
Peptide library designs
Peptides derived from the amino acid sequences of Tl8 and Tl9 were synthesized as 17-mers with 12 aa overlapping (JPT Peptide Technologies, Germany). Sixty-eight Tl8 peptides and 57 Tl9 peptides were tested. Peptides (approx. 67 nmol) were resuspended in RPMI in a 96 well format. Up to 8 peptides in the same row were pooled, and up to 10 peptides in the same column were pooled so that each peptide was present in both row and column pools and used for IFNγ assay. Peptide pools (1 μg ml-1 per peptide) were incubated with fibroblasts in 200 μl culture medium for IFNγ ELISA, and putative positive peptides were confirmed by peptide titration (0.01–10 μg ml-1) in the IFNγ assay. T cell epitope sequences were further examined by synthesizing 9–12-mer peptides of each epitope truncated sequentially either at the N or C terminal (JPT Peptide Technologies, Germany). Peptides were dissolved in up to 20% DMSO in PBS to 10 mg ml-1, then diluted in RPMI for IFNγ assays at 1–10 μg ml-1 as above.
Nucleotide sequences
Ovine MHC class I and II allele sequences were deposited in the European Nucleotide Archive with accession numbers LN868342 –LN868359 and HF954377 (Table 1). T. lestoquardi gene sequences were deposited in Genbank with accession numbers KT989585—KT989597 (Table 2).
Table 1. MHC class I and II alleles identified for individual sheep.
| Animal | Class I sequences (Accession number)a | Class II DRB (Accession number) | Reference |
|---|---|---|---|
| 1263 | Ovar-I*2K (LN868358), Ovar-I*U (LN868342), Ovar-I*2L (LN868359) | Ovar-DRB1*1802 homozygous (HF954377) | This study |
| 1343 | Ovar-I*V (LN868343), Ovar-I*W (LN868344), Ovar-I*X (LN868345) | Ovar-DRB1*0801, Ovar-DRB1*1201 | [39] |
| 1360 | Ovar-I*U (LN868342), Ovar-I*V (LN868343), Ovar-I*Y (LN868346), Ovar-I*Z (LN868347) | Ovar-DRB1*0501 homozygous | [39] |
| 4223 | Ovar-I*2A (LN868348), Ovar-I*2B (LN868349), Ovar-I*2C (LN868350), Ovar-I*2D (LN868351), Ovar-I*2E (LN868352), Ovar-I*2F (LN868353) | Ovar-DRB1*1201, Ovar-DRB1*0201 | [39] |
| 4247 | Ovar-I*2G (LN868354), Ovar-I*2H (LN868355), Ovar-I*2I (LN868356), Ovar-I*2J (LN868357) | Ovar-DRB1*1102, Ovar-DRB1*0702 | [39] |
a Local name assigned for partial mRNA sequence.
Table 2. Candidate genes for antigen screening.
| Gene product, size | TA orthologues, % ID | TP orthologues, % ID | Putative function of orthologues | GenBank accession no. | Reference |
|---|---|---|---|---|---|
| Tl1, 454 aa | TA17450, 305/532 (57.3%) | TP03_0849 (Tp1), 209/557 (37.5%) | Hypothetical protein | KT989585 | [17, 19] |
| Tl2, 177 aa | TA19865 (Ta2), 149/178 (83.7%) | TP01_0056 (Tp2), 109/177 (61.6%) | Surface protein d precursor | KT989586 | [17, 19] |
| Tl3, 264 aa | TA06115, 236/265 (89.1%) | TP01_0868, 198/266 (74.4%) | Hypothetical protein | KT989587 | [17] [19] |
| Tl5, 155 aa | TA14970 (Ta5), 154/155 (99.4%) | TP02_0767 (Tp5), 153/155 (98.7%) | Translation initiation factor eif-1A | KT989588 | [17, 19] |
| Tl6, 277 aa | TA19320, 273/277 (98.6%) | TP01_0188, 274/277 (98.9%) | Prohibitin | KT989589 | [17–19] |
| Tl7, 761 aa | TA12105, 706/723 (97.6%) | TP02_0244 (Tp7), 699/722 (96.8%) | Heat shock protein 90 | KT989590 | [17, 19] |
| Tl8, 413 a | TA11565, 382/413 (92.5%) | TP02_0140 (Tp8), 362/440 82.3% | Cysteine proteinase | KT989591 | [17, 19] |
| Tl9, 311 aa | TA15705 (Ta9), 188/344 (54.7%) | TP02_0895 (Tp9), 169/366 (46.2%) | Secreted protein in infected cell cytoplasm | KT989592 | [17, 19] |
| Tl10, 392 aa | TA10060, 377/448 (84.2%) | TP04_0772, 358/444 (80.6%) | Coronin | KT989593 | [18, 19] |
| Tl12, 858 aa | TA08425, 666/894 (74.5%) | TP04_0437, 472/945 (49.9%) | microneme-rhoptry antigen (p104) | KT989594 | [40] |
| Tl16, 275 aa | TA17315, 233/315 (74.0%) | TP04_0051, 176/488 (36.1%) | Surface protein precursor (TaSP or PIM) | KT989595 | [19, 41] |
| Tl13245, 1628 aa | TA13245, 1410/1669 (84.5%) | TP02_0052, 495/1644 (30.1%), TP02_0051 736/1635 (45.0%) | Hypothetical protein | KT989596 | [18] |
| Tl16020, 364 aa | TA16020, 277/370 (74.9%) | TP02_0952, 168/403 (41.7%) | Hypothetical protein | KT989597 | This study |
Results
Genotyping of MHC class I and II alleles
Class I and class II DRB1 allele expression for each of the five immunized animals were determined by sequencing of cloned PCR products. All identified class I sequences represented novel alleles with the exception of allele U, which was identified in an earlier unpublished study (Table 1). Comparison of the predicted amino acid sequences of the novel alleles to the reference sequence N*00301 shows regions of polymorphism, particularly where residues were predicted to interact with peptides (S1 Fig). Class II sequence based genotyping identified a new DRB1 allele (DRB1*1802) in animal 1263 and previously identified DRB1 alleles in the other animals; none of the alleles were shared between the five animals (Table 1).
Cytotoxic activity of CD8+ T cells from immunized sheep
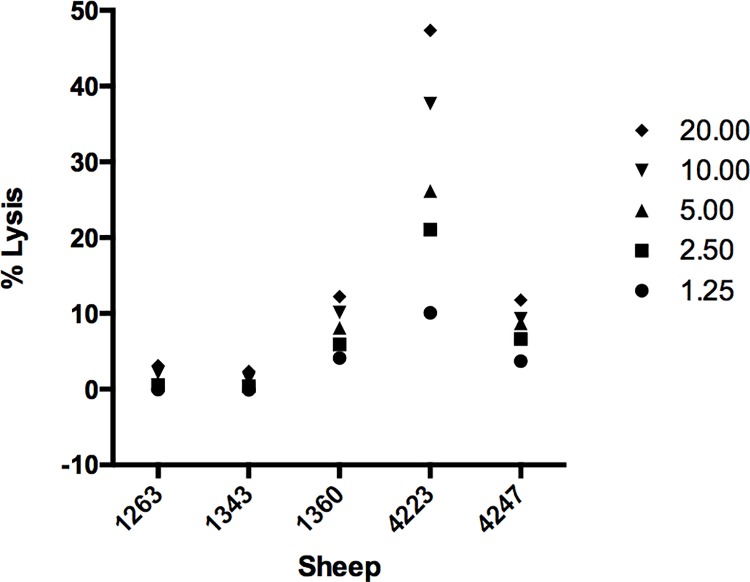
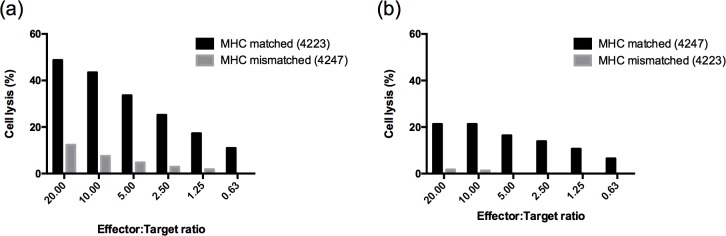
CD8+ T cell responses of sheep immunized with live parasites were examined by in vitro stimulation of PBMC with irradiated autologous parasitized cells and testing the responding cells in a cytotoxicity assay using the same infected cells as targets. CD8+ T cell lines were established from all five immunized sheep following two or three in vitro antigen stimulations and then enrichment for CD8+ T cells by complement-mediated lysis of CD4 T cells; CD8+ T cells were the predominant cell type (66.95 ± 0.014%) in these lines (S2 Fig). CD8+ T cells from each immunized animal were then tested for cytotoxicity against autologous T. lestoquardi-infected cells in a cytotoxicity assay. The CD8+ T cell lines exhibited variable levels of killing; the line from one animal gave a maximal killing of 47% and two lines gave lower levels of lysis (4–12%), while lysis by the remaining two lines was not significantly above background (Fig 1). Stimulated PBMC showed MHC-restricted cytotoxicity when assayed with autologous and unrelated infected cells (Fig 2).
Fig 1. Cytotoxic activity of CD8+ T cells from immunized sheep measured by indium oxine release assays.
Effector cells were stimulated twice with irradiated T. lestoquardi-infected cell lines and mixed with indium oxine labeled target T. lestoquardi-infected cells at indicated effector: target ratios.
Fig 2. MHC-specific cytotoxicity of stimulated PBMC.
All sheep samples were assayed similarly and two representative data are shown. (a) Sheep 4223 effectors lysed autologous infected cells more effectively than class I MHC-mismatched infected cells from Sheep 4247 at all effector: target ratios. (b) Sheep 4247 effectors lysed autologous infected cells, but not class I MHC mismatched infected cells from Sheep 4223.
Screening for T. lestoquardi antigens
A series of 13 T. lestoquardi candidate antigens were chosen to screen for recognition by the parasite-specific CD8+ T cell lines. They were selected based on orthology with T. parva and T. annulata antigens that were shown previously to be recognised by CD8+ T cells (Table 2), thus conforming to the premise that antigen recognition of T. lestoquardi in sheep is similar to that of T. annulata and T. parva in cattle. DNA sequences of the respective genes were obtained from a draft genome assembly of T. lestoquardi Lahr strain (W. Weir, unpublished data) and genes were obtained by PCR and sub-cloning the amplicons in recombinant plasmids.
Primary autologous fibroblast cell lines derived from the five sheep were transfected with recombinant plasmids incorporating each of the T. lestoquardi genes and screened for recognition by CD8+ T cells from the corresponding animal. Detection of a C-terminal V5 tag by immunofluorescence staining confirmed successful transfection of cells at 24 h and 48 h (S3 Fig).
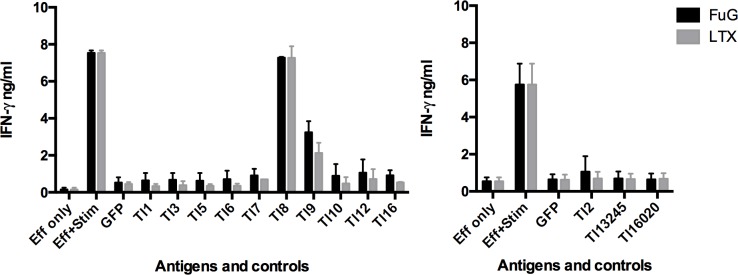
Measurement of IFNγ release by antigen-stimulated CD8+ T cells demonstrated that one of five animals (Sheep 4247) responded to two T. lestoquardi antigens, Tl8 and Tl9 (Fig 3). There was no detectable response to any other antigen by T cell lines from this sheep or the other 4 sheep (data not shown). The CD8+ T cell screens included transfection using two different reagents–Lipofectamine LTX and Fugene. Although transfection efficacy was not assessed for Fugene, both reagents yielded similar positive results (Fig 3).
Fig 3. CD8+ T cell response to 13 T. lestoquardi antigens measured by IFNγ ELISA.
Sheep 4247 fibroblasts were transfected with expression constructs of candidate antigen genes using either Lipofectamine LTX or Fugene transfection reagents for 48 h before the addition of effector cells for 72 h and IFNγ ELISA. The antigens were tested separately and data are presented as mean ± SD of biological repeats (n = 3).
Identification of CD8+ T cell epitopes
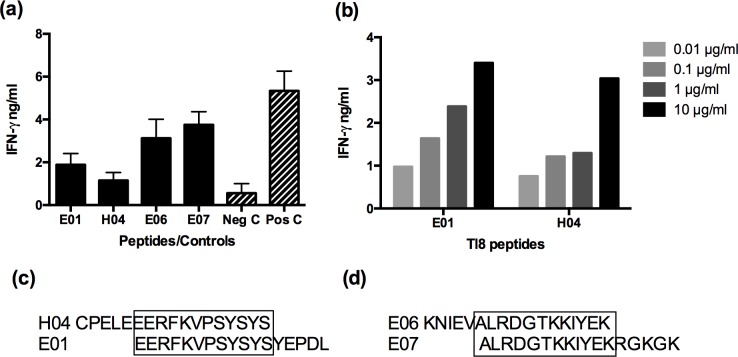
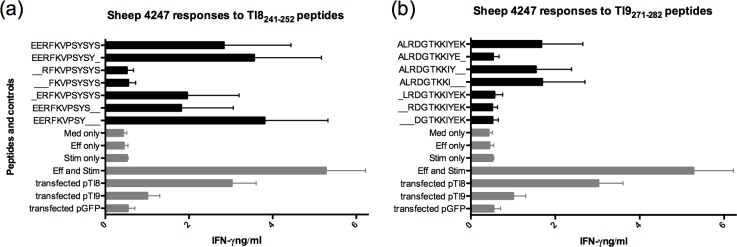
CD8+ T cells from sheep 4247, which recognised the Tl8 and Tl9 antigens, were tested for recognition of overlapping peptides spanning the full length of each protein. Positive results were obtained with two contiguous overlapping peptides from each antigen. These were peptides E01 and H04 of Tl8, and peptides E06 and E07 of Tl9 (Fig 4A). However, the IFNγ production responses detected with the Tl8 peptides at a concentration of 1 μg ml-1 were relatively weak. Further titration of the Tl8 peptides (at concentrations of 0.01–10 μg ml-1) (Fig 4B) demonstrated that a concentration of 10 μg ml-1 of Tl8 peptide was required to obtain an equivalent IFNγ response induced by the Tl9 peptides at a concentration of 1 μg ml-1. The overlapping peptide sequence of E01 and H04 of Tl8 was EERFKVPSYSYS, which corresponded to amino acid residues 241–252 (Fig 4C). The overlapping peptide sequence of E06 and E07 of Tl9 was ALRDGTKKIYEK, which corresponded to amino acid residues 271–282 (Fig 4D).
Fig 4. Tl8 and Tl9 peptide screen for CTL epitopes.
(a) Peptides positively identified from peptide pools were incubated at 1 μg ml-1 with fibroblasts and assayed for IFNγ production by autologous stimulated effector cells. T. lestoquardi-infected cells incubated with effectors served as a positive control and effectors only served as a negative control. (b) Tl8 peptides were titrated and assayed for IFNγ production by autologous stimulated effector cells, which increased with increasing concentrations of peptides. (c) Overlapping peptide sequence of H04 and E01 of Tl8. (d) Overlapping peptide sequence of E06 and E07 of Tl9.
In order to identify the definitive epitopes within the Tl8241-252 (EERFKVPSYSYS) and Tl9271-282 (ALRDGTKKIYEK) sequences, a series of truncated peptides ranging from 9 to 12 amino acids in length were tested for recognition by the respective CD8 T cell lines (Fig 5). For the Tl8241-252, removing the first N-terminal Glu residue reduced IFNγ response, but removing the first two N-terminal Glu residues completely abolished the response (Fig 5A). A Tyr residue appears to be equally important at the C-terminus, as truncated peptides with this residue removed elicited a poor response, whereas truncated peptides with a C-terminal Tyr249 or Tyr251 stimulated the highest responses (Fig 5A). The 9-mer (Tl8241-249 EERFKVPSY) resulted in the highest responses for all three experimental repeats, followed in performance by the 11-mer (Tl8241-251 EERFKVPSYSY) and the 12-mer (Tl8241-252 EERFKVPSYSYS) (Fig 5A). For Tl9, a C-terminal truncated 9-mer peptide was recognised as effectively as the full length 12 mer (Fig 5B). Removing one or more of the first 4 N-terminal residues (Ala, Lys, Try, Ileu) resulted in a complete lack of T cell recognition (Fig 5B). Hence the shortest antigenic peptide to induce a strong IFNγ response was the 9-mer Tl9271-279 ALRDGTKKI (Fig 5B).
Fig 5.
IFNγ response of antigenic epitopes of (a) Tl8241-252 (EERFKVPSYSYS) and (b) Tl9271-282 (ALRDGTKKIYEK). Different peptide sequence combinations ranging from 9-mer to 12-mer at 1 μg ml-1 were incubated for 24 h with fibroblasts before adding effector cells for 72 h. Cell supernatants were harvested for IFNγ assay (n = 2).
A comparison of the amino acid sequence of Tl8 to that of the T. annulata and T. parva orthologues Ta8 and Tp8 showed 87.65% of residues were similar. Tl8241-249 EERFKVPSY did not overlap with the previously identified epitope of Tp8379-387 CGAELNHFL [29] (S4 Fig). The sequence of the corresponding 9 amino acid regions in Ta8 and Tp8 each differed by only one amino acid residue from the Tl8 epitope sequence (S4A Fig). A similar comparison of the amino acid sequences of Tl9 to Ta9 and Tp9 revealed only 51.9% similarity, although the N-terminal predicted signal peptide was highly conserved (S4B Fig). The latter is consistent with previous comparisons of Ta9 and Tp9 [19]. The positions of the epitope in the Tl9 protein differed from those of previously identified epitopes in the T. annulata and T. parva orthologues (S4 Fig). In contrast to Tl8, the amino acid sequences of the corresponding 9 amino acid regions in Ta9 and Tp9 differed at 5 and 6 amino acid positions respectively from the Tl9 sequence.
Discussion
A subunit vaccine against T. lestoquardi is an attractive alternative strategy to live attenuated vaccines; however, there is limited available data on T. lestoquardi antigens and the immune responses against them. This is the first report of CD8+ T cells from immunized sheep having cytotoxic activity against T. lestoquardi-infected lymphocytes and identification of potential T. lestoquardi antigens that can be recognised by this CD8+ T cell response. In addition, the results confirm that orthologues of antigens that are recognised by the bovine CD8+ T cell response are also recognised by the ovine immune response, although the identified epitopes may vary.
While initial cytotoxicity assays lacked a control to confirm specificity against parasite-infected cells, subsequent IFNγ assays were carried out with enriched CD8+ T cells and included uninfected fibroblasts, confirming parasite-specific responses mediated by CD8+ T cells. The relatively low cytotoxic activity of the sheep 4247 CD8+ T cell line nonetheless having a robust IFNγ response to Tl8 and Tl9 indicates that levels of cytotoxicity do not necessarily reflect the CD8 T cell IFNγ response. This is similar to observations with CD8+ T cell lines generated from cattle immunized against T. annulata, where there was variability in cytotoxicity but a consistent and specific IFNγ response to antigens [30].
Tl8 is predicted as a cysteine proteinase and Tl9 has no known predicted function but is unique to transforming Theileria species; a Ta9/Tp9 orthologue was absent in the non-transforming species, T. orientalis [42]. Sequence analysis by SignalP 4.1 [43] did not predict a signal peptide in Tl8 or Ta8, while Tp8 has a predicted signal anchor peptide [17], suggesting that Tl8 and Ta8 may not be secreted proteins, while Tp8 may be a membrane protein. In contrast, Tl9 is predicted to contain a signal peptide, similar to Ta9 and Tp9. Ta9 has been reported to be secreted into the cytoplasm of macroschizont infected cells [19]. Tp8, Tp9 and Ta9 have been shown to be antigenic in cattle [17, 19, 29].
The detection of T cell responses to two of only thirteen candidate antigens, in one of five immunized sheep is a reasonable detection rate relative to previous antigen screening studies for T. parva and T. annulata [17, 19]. The data confirms that some parasite gene products serve as antigens in several Theileria species, and that T. lestoquardi antigens are recognized by CD8+ T cells from different animals in a preferential manner, as is the case for T. parva and T. annulata in cattle [19, 29, 31]. Previous studies of T. parva indicated that the latter reflects an effect of MHC type on antigenic dominance. In this regard, the MHC type of sheep 4247 differed from that of the other sheep examined. A high peptide concentration was required to detect the Tl8 epitope, which was likely due to peptide insolubility issues rather than a lack of reactivity per se, for the following reasons: a) RPMI was used for resuspension of peptide and precipitates were observed; b) specific T cells were present, as pTl8 transfected fibroblast controls produced high levels of IFNγ (5.3 ± 1.4 ng ml-1 for LTX and 3.9 ± 0.9 ng ml-1 for Fugene); c) BLASTP of the Tl8 epitope sequence against the translated sequence of the T. lestoquardi genome did not result in any high-scoring matches (other than Tl8), arguing against the possibility that the detected Tl8 response represented cross-reactivity with a true epitope in another protein [44].
For both Tl8 and Tl9, we determined that 9-mer peptides were optimal for inducing an IFNγ response. This length is consistent with T. parva antigenic peptides derived from Tp2, Tp4, Tp5, Tp7 and Tp8 in cattle, which are all 9-mers [29]. However, the peptide sequences did not overlap with previously identified antigenic epitope sites in the Tp8 and Ta9 proteins. This may be due to differences in the peptide binding sites of MHC I molecules of sheep and cattle, other antigen-dependent factors such as peptide conformations and unidentified epitopes, or a combination of unidentified MHC I molecules and epitopes. In this regard, prediction of T. parva and T. annulata epitopes binding to bovine MHC I (BoLA) molecules by NetMHCpan has generated a greater number of epitopes and binding BoLA molecules than peptide mapping or truncation assays [45, 46]. This tool could be useful for the prediction of additional putative T. lestoquardi epitopes when more data on ovine MHC I alleles becomes available.
Analysis of the MHC class I diversity in sheep has been limited to alleles from Scottish Blackface [39] and French Prealpe breeds (K. Ballingall, unpublished data). Therefore, it was unsurprising that most alleles identified in the Swaledale/Leicester cross sheep used in this study were novel and diverse. However, it is of interest that alleles 2G, H, I, J from the responding sheep 4247 have a unique polymorphic cluster corresponding to changes Phe113 and Met114 of the reference sequence N*00301; Met114 was predicted to bind peptides [33]. Allele 2K also possessed this unique cluster, although it is one of three alleles identified in an unresponsive sheep.
To summarize, we were able to identify two T. lestoquardi proteins that are recognised by a CD8+ T cell line established in vitro from an MHC I defined immune sheep, indication that they are involved in potentially protective T cells response in vivo against T. lestoquardi-infected leukocytes. Furthermore, we were able to deduce the minimal length peptides required for recognition of these antigens by a CD8+ T cells response. More comprehensive antigen screens and additional studies are necessary to determine the genetic diversity of both parasite and host, and to test the ability of identified proteins to induce protection against T. lestoquardi challenge. While the use of an attenuated T. lestoquardi-infected cell line has shown protection in one study [12], a more targeted approach to vaccination, ideally utilising antigens conserved between different strains of T. lestoquardi, has practical advantages in terms of safety, production, storage and administration.
Supporting Information
Conflicting residues are shown, consensus residues are indicated by dots, gaps are indicated by dashes, and MHC class I residues previously predicted to interact with peptides presented to T cells are indicated by asterisks. Residues are numbered according to N*00301 sequence.
(TIFF)
(TIF)
Expression was detected by antibodies against the C-terminal V5 tag (green). Cells were counterstained with DAPI (blue).
(TIF)
Sequence comparisons of (a) Tl8241-249 EERFKVPSY and (b) Tl9271-279 ALRDGTKKI to T. annulata and T. parva orthologues. Conflicting residues are in red, antigenic epitopes identified in this study and in previous studies (Tp8379-387 CGAELNHFL, Ta940-49 QRSPMFEGTL, and Ta964-72 SKFPKMRMG) are boxed in purple, predicted signal peptide sequences are indicated, and conserved residues within an epitope region are indicated by asterisks.
(TIFF)
(DOCX)
Acknowledgments
We thank the late Dirk A.E. Dobbelaere for contributing to the selection, cloning and expression of T. lestoquardi antigens. We thank the late Declan J. McKeever for his conceptualization, critical analysis and supervision of this work. We dedicate this publication to Declan J. McKeever, a highly respected and revered scientist, mentor, colleague and friend. This manuscript has been assigned the approval number PPB_01219 by the RVC.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was funded by the EU Seventh Framework Programme for Research (FP7), grant number 245145—PIROVAC, http://www.theileria.org/pirovac/. The funder had no role in study design, data collection and interpretation, or the decision to submit work for publication.
References
- 1.El Imam AH, Taha KM. Malignant ovine theileriosis (Theileria lestoquardi): a review. Jordan Journal of Biological Sciences. 2015;8(3):165–74. [Google Scholar]
- 2.Taha KM, Salih DA, Ahmed BM, Enan KA, Ali AM, Elhussein AM. First confirmed report of outbreak of malignant ovine theileriosis among goats in Sudan. Parasitology research. 2011;109(6):1525–7. Epub 2011/05/04. 10.1007/s00436-011-2428-y . [DOI] [PubMed] [Google Scholar]
- 3.Zakian A, Nouri M, Barati F, Kahroba H, Jolodar A, Rashidi F. Vertical transmission of Theileria lestoquardi in sheep. Veterinary parasitology. 2014;203(3–4):322–5. Epub 2014/05/13. 10.1016/j.vetpar.2014.04.007 . [DOI] [PubMed] [Google Scholar]
- 4.Taha KM, Elhussein AM. Experimental transmission of Theileria lestoquardi by developmental stages of Hyalomma anatolicum ticks. Parasitology research. 2010;107(4):1009–12. Epub 2010/07/08. 10.1007/s00436-010-1968-x . [DOI] [PubMed] [Google Scholar]
- 5.Abdigoudarzi M. Detection of naturally infected vector ticks (acari: ixodidae) by different species of babesia and theileria agents from three different enzootic parts of iran. Journal of arthropod-borne diseases. 2013;7(2):164–72. Epub 2014/01/11. ; PubMed Central PMCID: PMCPmc3875883. [PMC free article] [PubMed] [Google Scholar]
- 6.Razmi G, Pourhosseini M, Yaghfouri S, Rashidi A, Seidabadi M. Molecular detection of Theileria spp. and Babesia spp. in sheep and ixodid ticks from the northeast of Iran. The Journal of parasitology. 2013;99(1):77–81. Epub 2012/08/29. 10.1645/ge-3202.1 . [DOI] [PubMed] [Google Scholar]
- 7.Walker JG, Klein EY, Levin SA. Disease at the wildlife-livestock interface: acaricide use on domestic cattle does not prevent transmission of a tick-borne pathogen with multiple hosts. Veterinary parasitology. 2014;199(3–4):206–14. Epub 2013/12/10. 10.1016/j.vetpar.2013.11.008 . [DOI] [PubMed] [Google Scholar]
- 8.El Hussein AM, El Ghali A, Mohammed SA. Efficacy of buparvaquone in the treatment of malignant theileriosis of sheep in Ed-Damaar province N. State, Sudan. A field trial. The Sud J Vet Res. 1993;12:51–7. [Google Scholar]
- 9.Zia-ur-Rehman, Khan MS, Avais M, Aleem M, Shabbir MZ, Khan JA. Prevalence of Theileriosis in Sheep in Okara District, Pakistan. Pakistan J Zool. 2010;42(5):639–43. [Google Scholar]
- 10.Hawa NJ, Latif BM, Ali SR. Immunization of sheep against Theileria hirci infection with schizonts propagated in tissue culture. Veterinary parasitology. 1981;9(2):91–7. Epub 1981/12/01. . [DOI] [PubMed] [Google Scholar]
- 11.Hooshmand-Rad P. The use of tissue culture attenuated live vaccine for Theileria hirci. Developments in biological standardization. 1985;62:119–27. Epub 1985/01/01. . [PubMed] [Google Scholar]
- 12.Ahmed BM, Taha KM, Enan KA, Elfahal AM, El Hussein AR. Attenuation of Theileria lestoquardi infected cells and immunization of sheep against malignant ovine theileriosis. Vaccine. 2013;31(42):4775–81. Epub 2013/08/21. 10.1016/j.vaccine.2013.08.004 . [DOI] [PubMed] [Google Scholar]
- 13.Shkap V, de Vos AJ, Zweygarth E, Jongejan F. Attenuated vaccines for tropical theileriosis, babesiosis and heartwater: the continuing necessity. Trends in parasitology. 2007;23(9):420–6. Epub 2007/07/28. 10.1016/j.pt.2007.07.003 . [DOI] [PubMed] [Google Scholar]
- 14.Ali AM, Beyer D, Bakheit MA, Kullmann B, Salih DA, Ahmed JS, et al. Influence of subculturing on gene expression in a Theileria lestoquardi-infected cell line. Vaccine. 2008;26 Suppl 6:G17–23. Epub 2009/01/31. 10.1016/j.vaccine.2008.10.009 . [DOI] [PubMed] [Google Scholar]
- 15.Hall R, Ilhan T, Kirvar E, Wilkie G, Preston PM, Darghouth M, et al. Mechanism(s) of attenuation of Theileria annulata vaccine cell lines. Tropical medicine & international health: TM & IH. 1999;4(9):A78–84. Epub 1999/10/30. . [DOI] [PubMed] [Google Scholar]
- 16.Shkap V, Pipano E, Rasulov I, Azimov D, Savitsky I, Fish L, et al. Proteolytic enzyme activity and attenuation of virulence in Theileria annulata schizont-infected cells. Veterinary parasitology. 2003;115(3):247–55. Epub 2003/08/26. . [DOI] [PubMed] [Google Scholar]
- 17.Graham SP, Pelle R, Honda Y, Mwangi DM, Tonukari NJ, Yamage M, et al. Theileria parva candidate vaccine antigens recognized by immune bovine cytotoxic T lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(9):3286–91. Epub 2006/02/24. 10.1073/pnas.0511273103 ; PubMed Central PMCID: PMCPmc1413922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiels B, Langsley G, Weir W, Pain A, McKellar S, Dobbelaere D. Alteration of host cell phenotype by Theileria annulata and Theileria parva: mining for manipulators in the parasite genomes. International journal for parasitology. 2006;36(1):9–21. Epub 2005/10/14. 10.1016/j.ijpara.2005.09.002 . [DOI] [PubMed] [Google Scholar]
- 19.MacHugh ND, Weir W, Burrells A, Lizundia R, Graham SP, Taracha EL, et al. Extensive polymorphism and evidence of immune selection in a highly dominant antigen recognized by bovine CD8 T cells specific for Theileria annulata. Infection and immunity. 2011;79(5):2059–69. Epub 2011/02/09. 10.1128/iai.01285-10 ; PubMed Central PMCID: PMCPmc3088144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leemans I, Hooshmand-Rad P, Uggla A. The indirect fluorescent antibody test based on schizont antigen for study of the sheep parasite Theileria lestoquardi. Veterinary parasitology. 1997;69(1–2):9–18. Epub 1997/04/01. . [DOI] [PubMed] [Google Scholar]
- 21.Bakheit M, Scholzen T, Ahmed JS, Seitzer U. Identification of potential antigenic proteins of Theileria lestoquardi. Annals of the New York Academy of Sciences. 2006;1081:463–4. Epub 2006/12/01. 10.1196/annals.1373.065 . [DOI] [PubMed] [Google Scholar]
- 22.Bakheit MA, Scholzen T, Ahmed JS, Seitzer U. Molecular characterization of a Theileria lestoquardi gene encoding for immunogenic protein splice variants. Parasitology research. 2006;100(1):161–70. Epub 2006/08/10. 10.1007/s00436-006-0255-3 . [DOI] [PubMed] [Google Scholar]
- 23.Leemans I, Fossum C, Johannisson A, Hooshmand-Rad P. Comparative studies on surface phenotypes of Theileria lestoquardi and T. annulata schizont-infected cells. Parasitology research. 2001;87(9):768–77. Epub 2001/09/26. . [DOI] [PubMed] [Google Scholar]
- 24.Sparagano OA, Spitalska E, Namavari M, Torina A, Cannella V, Caracappa S. Phylogenetics of Theileria species in small ruminants. Annals of the New York Academy of Sciences. 2006;1081:505–8. Epub 2006/12/01. 10.1196/annals.1373.075 . [DOI] [PubMed] [Google Scholar]
- 25.Seitzer U, Ahmed J. Tropical theileriosis: cytotoxic T lymphocyte response to vaccination. Vaccine. 2008;26 Suppl 6:G24–8. Epub 2009/01/31. 10.1016/j.vaccine.2008.10.039 . [DOI] [PubMed] [Google Scholar]
- 26.Morrison WI. Progress towards understanding the immunobiology of Theileria parasites. Parasitology. 2009;136(12):1415–26. Epub 2009/08/21. 10.1017/s0031182009990916 . [DOI] [PubMed] [Google Scholar]
- 27.McKeever DJ, Taracha EL, Innes EL, MacHugh ND, Awino E, Goddeeris BM, et al. Adoptive transfer of immunity to Theileria parva in the CD8+ fraction of responding efferent lymph. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(5):1959–63. Epub 1994/03/01. ; PubMed Central PMCID: PMCPmc43284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taracha EL, Awino E, McKeever DJ. Distinct CD4+ T cell helper requirements in Theileria parva-immune and -naive bovine CTL precursors. Journal of immunology (Baltimore, Md: 1950). 1997;159(9):4539–45. . [PubMed] [Google Scholar]
- 29.Graham SP, Pelle R, Yamage M, Mwangi DM, Honda Y, Mwakubambanya RS, et al. Characterization of the fine specificity of bovine CD8 T-cell responses to defined antigens from the protozoan parasite Theileria parva. Infection and immunity. 2008;76(2):685–94. Epub 2007/12/12. 10.1128/iai.01244-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machugh ND, Burrells AC, Morrison WI. Demonstration of strain-specific CD8 T cell responses to Theileria annulata. Parasite Immunol. 2008;30(8):385–93. Epub 2008/05/24. 10.1111/j.1365-3024.2008.01038.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacHugh ND, Connelley T, Graham SP, Pelle R, Formisano P, Taracha EL, et al. CD8+ T-cell responses to Theileria parva are preferentially directed to a single dominant antigen: Implications for parasite strain-specific immunity. European journal of immunology. 2009;39(9):2459–69. Epub 2009/08/12. 10.1002/eji.200939227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hooshmand-Rad P, Magnusson U, Uggla A. Some characteristics of ovine lymphoid cells infected in vivo by Theileria hirci. Parasitology research. 1993;79(3):195–9. Epub 1993/01/01. . [DOI] [PubMed] [Google Scholar]
- 33.Miltiadou D, Ballingall KT, Ellis SA, Russell GC, McKeever DJ. Haplotype characterization of transcribed ovine major histocompatibility complex (MHC) class I genes. Immunogenetics. 2005;57(7):499–509. Epub 2005/07/20. 10.1007/s00251-005-0008-y . [DOI] [PubMed] [Google Scholar]
- 34.Ballingall KT, Tassi R. Sequence-based genotyping of the sheep MHC class II DRB1 locus. Immunogenetics. 2010;62(1):31–9. Epub 2009/11/28. 10.1007/s00251-009-0410-y . [DOI] [PubMed] [Google Scholar]
- 35.Emery DL, Morrison WI. Generation of autologous mixed leucocyte reactions during the course of infection with Theileria parva (East Coast Fever) in cattle. Immunology. 1980;40(2):229–37. Epub 1980/06/01. ; PubMed Central PMCID: PMCPmc1457984. [PMC free article] [PubMed] [Google Scholar]
- 36.Jeong JY, Yim HS, Ryu JY, Lee HS, Lee JH, Seen DS, et al. One-step sequence- and ligation-independent cloning as a rapid and versatile cloning method for functional genomics studies. Appl Environ Microbiol. 2012;78(15):5440–3. Epub 2012/05/23. 10.1128/aem.00844-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hibbs AR. Confocal Microscopy for Biologists 1st ed: Springer; 2004. 484 p. [Google Scholar]
- 38.Goddeeris BM, Morrison WI, Teale AJ. Generation of bovine cytotoxic cell lines, specific for cells infected with the protozoan parasite Theileria parva and restricted by products of the major histocompatibility complex. European journal of immunology. 1986;16(10):1243–9. Epub 1986/10/01. 10.1002/eji.1830161010 . [DOI] [PubMed] [Google Scholar]
- 39.Ballingall KT, Herrmann-Hoesing L, Robinson J, Marsh SG, Stear MJ. A single nomenclature and associated database for alleles at the major histocompatibility complex class II DRB1 locus of sheep. Tissue Antigens. 2011;77(6):546–53. Epub 2011/03/03. 10.1111/j.1399-0039.2011.01637.x . [DOI] [PubMed] [Google Scholar]
- 40.Woods KL, Theiler R, Muhlemann M, Segiser A, Huber S, Ansari HR, et al. Recruitment of EB1, a master regulator of microtubule dynamics, to the surface of the Theileria annulata schizont. PLoS pathogens. 2013;9(5):e1003346 Epub 2013/05/16. 10.1371/journal.ppat.1003346 ; PubMed Central PMCID: PMCPmc3649978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seitzer U, Gerber S, Beyer D, Dobschanski J, Kullmann B, Haller D, et al. Schizonts of Theileria annulata interact with the microtubuli network of their host cell via the membrane protein TaSP. Parasitology research. 2010;106(5):1085–102. Epub 2010/02/18. 10.1007/s00436-010-1747-8 . [DOI] [PubMed] [Google Scholar]
- 42.Hayashida K, Hara Y, Abe T, Yamasaki C, Toyoda A, Kosuge T, et al. Comparative genome analysis of three eukaryotic parasites with differing abilities to transform leukocytes reveals key mediators of Theileria-induced leukocyte transformation. mBio. 2012;3(5):e00204–12. Epub 2012/09/07. 10.1128/mBio.00204-12 ; PubMed Central PMCID: PMCPmc3445966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature methods. 2011;8(10):785–6. Epub 2011/10/01. 10.1038/nmeth.1701 . [DOI] [PubMed] [Google Scholar]
- 44.Macdonald IK, Harkiolaki M, Hunt L, Connelley T, Carroll AV, MacHugh ND, et al. MHC class I bound to an immunodominant Theileria parva epitope demonstrates unconventional presentation to T cell receptors. PLoS pathogens. 2010;6(10):e1001149 Epub 2010/10/27. 10.1371/journal.ppat.1001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen AM, Rasmussen M, Svitek N, Harndahl M, Golde WT, Barlow J, et al. Characterization of binding specificities of bovine leucocyte class I molecules: impacts for rational epitope discovery. Immunogenetics. 2014;66(12):705–18. Epub 2014/09/05. 10.1007/s00251-014-0802-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svitek N, Hansen AM, Steinaa L, Saya R, Awino E, Nielsen M, et al. Use of "one-pot, mix-and-read" peptide-MHC class I tetramers and predictive algorithms to improve detection of cytotoxic T lymphocyte responses in cattle. Veterinary research. 2014;45:50 Epub 2014/04/30. 10.1186/1297-9716-45-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Conflicting residues are shown, consensus residues are indicated by dots, gaps are indicated by dashes, and MHC class I residues previously predicted to interact with peptides presented to T cells are indicated by asterisks. Residues are numbered according to N*00301 sequence.
(TIFF)
(TIF)
Expression was detected by antibodies against the C-terminal V5 tag (green). Cells were counterstained with DAPI (blue).
(TIF)
Sequence comparisons of (a) Tl8241-249 EERFKVPSY and (b) Tl9271-279 ALRDGTKKI to T. annulata and T. parva orthologues. Conflicting residues are in red, antigenic epitopes identified in this study and in previous studies (Tp8379-387 CGAELNHFL, Ta940-49 QRSPMFEGTL, and Ta964-72 SKFPKMRMG) are boxed in purple, predicted signal peptide sequences are indicated, and conserved residues within an epitope region are indicated by asterisks.
(TIFF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.