To the Editor
Older adults with dementia are at a higher risk of falling, with an annual incidence of approximately 60% to 80%,1 at least twice that of cognitively normal older adults,2 ultimately leading to institutionalization, fractures, and mortality due to falls.3 Although the reason for the high incidence of falls in elderly people with dementia remains unclear, an important related factor is their limited attentional resource allocation while walking.1 Attention is a necessary cognitive resource for normal gait, and impairments in attention and executive function are associated with greater risk of falls in this population.4,5
Recently, it has been suggested that acetylcholine esterase (AChE) inhibitors, a cognitive enhancer medication for symptomatic treatment of Alzheimer’s disease (AD), may improve gait performance.6 Because AChE inhibitors are known to improve attention and executive function in AD,7 it was hypothesized that AChE inhibitors would reduce fall risk in people with AD by increasing their gait velocity and reducing their gait variability. Gait variability quantifies the automaticity of gait, and higher variability reflects less rhythmicity, expressing a more instable gait pattern.8 The effect of AchE inhibitors on gait velocity and gait variability, both well-established predictors of falls,4,9 has not been evaluated.
METHODS
With local institutional review board approval, using an open-label design, the effect of donepezil on gait performance was tested in elderly people with mild AD. Their gait changes were compared with those of control subjects with mild cognitive impairment (MCI). In the AD group, gait velocity (cm/s) and stride time (ms) were determined before intervention, after 1 month with 5 mg/day of donepezil, and after 3 months with 10 mg/day of donepezil. The total follow-up period was 4 months. In the MCI (control) group, gait variables were evaluated at baseline and after 6 months of follow-up. Gait was assessed while single-tasking (only walking, sG) and while dual-tasking (walking while counting backwards from 100, dG) using an electronic walkway (GAITRite Systems Inc., Havertown, PA). Subjects with depression, with orthopedic diagnoses affecting gait, using walking aids, or using sedative medication were excluded. Gait variability was evaluated as stride time variability and reported as coefficient of variation (CV =standard deviation/mean × 100).8 Before and after treatment comparisons were performed with the Wilcoxon matched-pairs signed-ranks test and comparisons between groups with the Mann-Whitney test. P<.05 was considered significant.
RESULTS
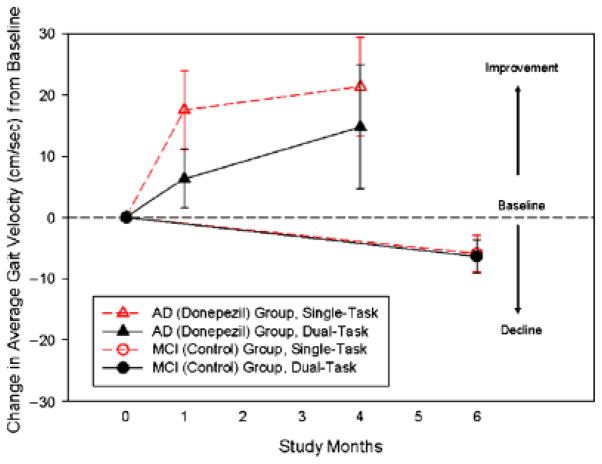
Six subjects with mild AD (67% women, mean age 79.9 ± 4.8, Mini-Mental State Examination (MMSE) score 22.3 ± 1.2, mean Montreal Cognitive Assessment (MoCA) score 15 ± 1.4) and eight no-treatment control subjects with MCI (64% women, mean age 75.6 ± 6.2, mean MMSE score 27.9 ± 1.7, mean MoCA score 22.9 ± 1.7) were assessed. Participants with AD taking donepezil increased their mean gait velocity after 1 month (single-task gait velocity from 89.7 ± 11 to 106.9 ± 22 cm/s, P =.045; dual-task gait velocity from 78.2 ± 22 to 89.7 ± 13 cm/s, P =.047) under both walking conditions. As shown in Figure 1, these increments had improved 4 months after baseline by 21.4 ± 16.2 cm/s for single-task gait velocity and by 14.8 ± 20.2 cm/s for dual-task gait velocity (P =.04). Gait variability decreased under both conditions during follow-up (CV single-task from 22.3% to 11.30%, P =.04; CV dual-task from 28.3% to 27.8%, P =.09). By contrast, participants with MCI decreased their mean gait velocity (single-task gait velocity from 112.5 ± 22 to 106.5 ± 14 cm/s, P =.08, dual-task gait velocity from 114.8 ± 11.5 to 108 ± 43, P =.09, Figure 1) and increased their gait variability (CV single task from 7.57% to 9.74%, P =.08; CV dual task from 9.24% to 12.36%, P =.07). Between-group differences showed significantly less gait variability in the AD group (P =.046) than in the MCI group.
Figure 1.
Mean and standard error changes from baseline through follow-up on gait velocity during single and dual tasking in Alzheimer’s disease (AD) (donepezil) and mild cognitive impairment MCI (control).
DISCUSSION
This study provides evidence that AchE inhibitors may reduce falls risk in people with mild AD. Donepezil treatment significantly increased gait velocity and reduced gait variability, resulting in a more-stable walking pattern in the intervention group. These improvements were found early, after 1 month of intervention, and they were sustained for 4 months, suggesting a dose-response pattern (Figure 1). This effect was slightly more important during single tasking, showing that dual tasking affects cortical control of gait. By contrast, the control group experienced a decline in gait velocity over time and an increase in gait variability.
Because most of the studies targeting falls in people with dementia have been unable to prevent falls,10 it may be possible that this lack of effectiveness reflects different underlying mechanisms in falls risk factors. Although much is known about the multifactorial nature of falls, the number of falls and related injuries in people with dementia continues to increase. By characterizing and understanding the effects of cognitive enhancers on gait, an additional approach for reducing falls in this fast-growing population can be reached. These findings offer support and rationale to assess the effect of AchE inhibitors on gait performance and risk of falling in a larger, controlled clinical trial.
Acknowledgments
We are grateful for the thoughtful review from Dr. Denise Goens, Clinical Research Office, University of Western Ontario. We also thank Ms. Maggie Hall and Mr. Kevin Hansen for their help in the data gathering and analysis.
Footnotes
Author Contributions: Study concept and design: MMO. Acquisition of subjects and data: MMO, MB, JW. Analysis and interpretation of data: MMO. Preparation of manuscript: MMO. Critical review of manuscript: MMO, JW, MB.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this letter. Dr Montero-Odasso is a recipient of the Schulich Clinician-Scientist Award (2008–2011). This study was funded by a research grant from Lawson Health Research Institute at London, ON, Canada.
Sponsor’s Role: The funding sources had no role in the design, methodology, data analysis, or preparation of this manuscript.
References
- 1.Shaw FE. Prevention of falls in older people with dementia. J Neural Transm. 2007;114:1259–1264. doi: 10.1007/s00702-007-0741-5. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC, Rubin EH, Morris EJ, et al. Senile dementia of the Alzheimer’s type: An important risk factor for serious falls. J Gerontol. 1987;42:412–417. doi: 10.1093/geronj/42.4.412. [DOI] [PubMed] [Google Scholar]
- 4.Sheridan PL, Solomont J, Kowall N, et al. Influence of executive function on locomotor function: Divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc. 2003;51:1633–1637. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- 5.Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: Executive dysfunction contributes to fall risk in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:125–137. doi: 10.1159/000105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assal F, Allali G, Kressig RW, et al. Galantamine improves gait performance in patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56:946–947. doi: 10.1111/j.1532-5415.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- 7.Seltzer B, Zolnouni P, Nunez M, et al. Efficacy of donepezil in early-stage Alzheimer disease: A randomized placebo-controlled trial. Arch Neurol. 2004;61:1852–1856. doi: 10.1001/archneur.61.12.1852. [DOI] [PubMed] [Google Scholar]
- 8.Hausdorff JM. Gait variability: Methods, modeling and meaning. J Neuroeng Rehabil. 2005;2:19. doi: 10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60:1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 10.Oliver D, Connelly JB, Victor CR, et al. Strategies to prevent falls and fractures in hospitals and care homes and effect of cognitive impairment: Systematic review and meta-analyses. BMJ. 2007;334:82. doi: 10.1136/bmj.39049.706493.55. [DOI] [PMC free article] [PubMed] [Google Scholar]