Abstract
Perineuronal nets (PNNs) are enigmatic structures composed of extracellular matrix molecules that encapsulate the soma, dendrites, and axon segments of neurons in a lattice-like fashion. Although the majority of PNNs condense around parvalbumin-expressing GABA(γ-aminobutyric acid)ergic interneurons, some glutamatergic pyramidal cells in the brain are also surrounded by PNNs. Experimental findings suggest pivotal roles of PNNs in the regulation of synaptic formation and function. There has also been an increasing body of evidence linking PNN abnormalities to schizophrenia. Interestingly, the number of PNNs progressively increases during postnatal development until plateauing around the period of late adolescence and early adulthood, which temporally coincides with the age of onset of schizophrenia. Given the established role of PNNs in modulating developmental plasticity, the PNN represents a possible candidate for altering the onset and progression of schizophrenia. Similarly, the reported function of PNNs in regulating the trafficking of glutamate receptors places them in a critical position to modulate synaptic pathology, considered a cardinal feature of schizophrenia. Here we discuss the physiological role of PNNs in neural function, synaptic assembly and plasticity in addition to how they interface with circuit/system mechanisms of cognition. An integrated understanding of these neurobiological processes should provide a better basis to elucidate how PNN abnormalities influence brain function and contribute to the pathogenesis of neurodevelopmental disorders such as schizophrenia.
Keywords: Critical Period, Neurodevelopment, Parvalbumin Interneurons, Perineuronal Nets, Schizophrenia, Synaptic Plasticity
Schizophrenia is a multi-factorial disorder of neurodevelopmental origin (1-4) thought to arise from a complex interaction between genetic and environmental factors (5-11). The onset of schizophrenia occurs during late adolescence or early adulthood, when brain circuitry involving the prefrontal cortex and hippocampus, in addition to other limbic regions, undergoes maturation (2-4). The pathophysiologic features of schizophrenia stem from a variety of aberrant neurobiological underpinnings that ultimately impinge on synaptic plasticity and synaptic connectivity (12-14), including anomalies in cortical myelogenesis (13) and synaptic pruning (12, 15), altered glutamatergic signalling (16-18) and reduced neuropil (19) in conjunction with atypical development of cortical inhibitory circuits (14, 20, 21), dopaminergic pathways (22) and perineuronal nets (PNNs) (23-31).
The PNN is a reticular structure of the neural extracellular matrix (ECM) found surrounding many neurons in the central nervous system (CNS) (32-34). The developmental pattern of increasing PNN formation corresponds to the ending of the “critical period”, a time window of postnatal life which is critical for experience-dependent formation of synaptic connections and wiring of functionally related neuronal pathways that underlie sensory, motor, cognitive, social, and language abilities (35), deficits in which have been linked to schizophrenia (36). Interestingly, experimental disruption of PNNs in the adult brain can reopen critical periods, and therefore, the PNN is generally considered to play a role in restricting synaptic plasticity (37). More recent work has expanded the list of functions attributed to PNNs within the CNS (Table 1), with potential significance to neuropathogenesis.
Table 1.
Summary of functions of perineuronal nets in the central nervous system
| Function influenced by PNNs | References |
|---|---|
| Cognitive Functions | |
| Audition | (85, 87) |
| Learning and Memory | (41, 51, 52, 54, 80) |
| Motor Coordination | (52) |
| Nociception | (86) |
| Olfaction | (38, 76) |
| Vision | (71) |
| Vocal Development | (49) |
| Neurophysiological/Cell Biological Functions | |
| Critical Period Regulation | (37, 61, 62, 67) |
| Glutamate Receptor Trafficking | (33, 89) |
| Intercellular Transport of molecules | (71) |
| Ion Homeostasis | (145-147) |
| Neuroprotection | (148, 149) |
| Membrane Compartmentalization | (89) |
| Regulation of γ-Oscillations | (25) |
| Synaptic stability and Plasticity | (32, 37, 150) |
Within this review we focus on the neurobiological functions of PNNs and their putative basis in schizophrenia pathophysiology. In addition, we expound on the emerging hypothesis linking a dysfunctional orthodenticle homeobox 2 (OTX2)-PNN interaction to the cognitive and cortical plasticity deficits associated with schizophrenia. We conclude by touching on how these observations may provide a neurobiological framework for the conceptualization of a molecular targeted intervention against this extremely debilitating condition.
Characteristics of PNNs: Molecular Heterogeneity, Distribution and Developmental Expression
PNNs are enriched in complex sugars called glycosaminoglycans (GAGs) and constitute a highly organized structure comprised of hyaluronan, link proteins, tenascin-R (TN-R) as well as chondriotin sulfate proteoglycans (CSPGs) - primarily the lectican family (aggrecan, brevican, neurocan and versican) and phosphocan (38-40). The possibility of simultaneously co-staining for various markers of the PNN has revealed a molecular heterogeneity of PNNs in distinct interneuronal subpopulations as indicated by different staining intensities of these markers in the cerebral cortex, subcortical forebrain and brainstem (41, 42). On the basis that PNNs exhibit a high degree of constitutive heterogeneity, it should be kept in mind that the alteration of a single marker during development or in disease states may not necessarily reflect the alteration or loss of the entire structure.
Previous research conducted across a variety of animal species (i.e., rhesus monkey (43, 44), bison (45), dog (46), gerbil (47), guinea pig (48), zebra finch (49), rat (38), and mouse (25, 50-56)) has shown PNNs to be widely distributed in the brain. Similarly, in humans, PNNs have been found to be present in a variety of brain regions, including entorhinal cortex (28), amygdala (28, 29, 57), hippocampus (58), motor and somatosensory cortex (59), visual cortex (27) and prefrontal cortex (27), all regions (bar the visual cortex) which have been reported to be affected in schizophrenia (60).
Experimental evidence from animal studies suggests a progressive increase in PNN expression to be associated with the postnatal maturation of the CNS (35, 51, 61-63). Consistent with the animal findings, a recent study using human postmortem brain tissue revealed that the number of PNNs in the prefrontal cortex also increases through the peripubertal period until late adolescence and early adulthood (27), which interestingly is considered the peak period of risk for onset of schizophrenia (2, 64). Indeed, notable findings from the visual (65), motor (66), and somatosensory system (67) in animals, as well as in the pallial (cortical) song nuclei (49) in songbirds suggests that PNN expression is dependent on neuronal activity during the critical period.
Although the majority of PNNs condense around the fast-spiking parvalbumin-expressing GABA(γ-aminobutyric acid)ergic interneurons, some pyramidal cells are also surrounded by PNNs (68, 69). The presence of PNNs around parvalbumin interneurons is of particular significance in the context of critical period plasticity. This period of plasticity seems to be triggered by a shift in the excitatory/inhibitory balance associated with the maturation of parvalbumin interneurons (35, 70), whose functional disturbances have been strongly linked to schizophrenia [25]. It is of note that parvalbumin interneurons control the initiation and termination of the developmental critical periods with termination being dependent on the formation of PNNs (35, 37, 71). In this context, the recent evidence showing the PNN to play a role in the “capture” of the homeodomain transcription factor OTX2 is of particular interest (71, 72). The presence of a short motif within the OTX2 sequence (RKQRRERTTFTRAQL), which partially overlaps with the first helix of the homeodomain, possesses consensus traits of a GAG-binding domain (73) and is a requisite for the specific recognition of OTX2 by parvalbumin interneurons that are surrounded by PNNs (71). The sulfation pattern of PNNs represents another important factor in OTX2 binding (61). Importantly, secretion of OTX2 from choroid plexus epithelial cells has been shown to signal the maturation of the parvalbumin interneurons and the subsequent regulation of critical period plasticity (71, 74). Therefore, PNNs do not only serve as “molecular brakes” that limit morphological and physiological plasticity, but also as “receptors” which function to control the availability of molecular factors (e.g., OTX2) that regulate plasticity and modulate parvalbumin cell function in order to influence the opening and possibly also the closure of the critical period (61, 71, 72, 74, 75).
The Role of the PNN in the Modulation of Cognitive Function
Given that PNN-encapsulated neurons are prevalent throughout the limbic system (28, 29, 76) and PNNs are capable of modulating receptors (i.e., α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, AMPA receptors) known to be integral for learning and memory (77), these structures have been considered to play an important role in memory formation (54), which may include emotional memory (51, 55) in addition to reward-related memory (50, 78). In fact, it has been reported that PNNs are involved in long-term potentiation (LTP) and long-term depression (LTD) in hippocampal slices, as these physiological events are impaired following enzymatic degradation of CSPGs, or removal of TN-R (79, 80). Moreover, PNNs seem to be important for the maturation and stabilization of synapses (65, 81), key developmental processes that are altered in schizophrenia (12-14), suggesting that PNNs also serve a specialized role for normal neurophysiological development.
The presence of PNNs in the striatum (52), olfactory pathway (38, 76), basal ganglia (82), cerebellum (83), thalamus (56), visual cortex (27), insular cortex (50), high vocal center (49), orbital cortex (53), central auditory pathway (43, 84, 85), and spinal cord (86) suggests that the regulatory effects of PNNs are involved in a wide range of brain functions, including motor coordination, olfaction, procedural learning, voluntary motor movement, arousal state, vision, integration of cognitive, affective, sensory and autonomic information, vocal development, mediating decision making, processing of auditory stimuli, and nociception, respectively. Indeed, depletion or alterations in the molecular composition of PNNs induced by enzyme degradation (such as chondroitinase ABC, ChABC), by knock-out methods, or by clinical disease processes are associated with anomalies in sensory perception (67), impaired vision (71) as well as altered gait (52). Conversely and perhaps surprisingly, global disruption of PNNs has been shown to significantly improve spatial learning (52), cognitive flexibility (87), recognition memory (54) and extinction training (51, 55). The neurobiological mechanism underlying these cognitive changes involves the PNNs that surround parvalbumin interneurons. In this regard, an increase in the number of inhibitory synapses impinging on parvalbumin interneurons has been associated with memory acquisition (88). This increase in inhibitory synapses on parvalbumin interneurons decreases their production of GABA and thereby increases cortical excitability. Interestingly, treatment with ChABC has been shown to induce a similar effect by increasing the number of inhibitory interneurons associated with parvalbumin interneurons (88). Taken together, these observations suggest that depletion of PNNs can increase the number of synaptic connections and plasticity, facilitating cognitive ability by altering the rules of underlying, experience-driven synaptic plasticity.
The neuronal surface is compartmentalized by PNNs, with these structures serving as lateral diffusion barriers for AMPA receptors and limiting synaptic exchange (89). Research evidence indicates that desensitized synaptic AMPA receptors can be exchanged for naïve receptors during high frequency firing, thereby increasing synaptic fidelity (33, 89, 90). Given that AMPA receptors are the main transducers of rapid excitatory transmission in the mammalian CNS, trafficking of these receptors is important for effective synaptic efficiency (via LTP and LTD) and subsequent memory formation (91). Thus, the role of PNNs in regulating the trafficking of AMPA receptors is critical for optimal brain health and function.
On the Potential Importance of the PNN in Schizophrenia
In this section, we describe postmortem and preclinical studies that may shed light on the role that PNNs may play in schizophrenia (23, 24, 26).
PNN Regulation of Synaptic Functions
Based on the observations that PNNs enwrap the somatodendritic axis and proximal dendrites of neurons, it has been suggested that PNNs play a role in the regulation of synaptic functions (33). Therefore, PNN disruption could affect the activity of intrinsic circuitry and consequent information outflow. Recent evidence stemming from a rodent model is consistent with this possibility. In this study, PNN degradation by ChABC restricted to the ventral hippocampus resulted in an increased activity of pyramidal neurons, postulated to result from disruption of GABAergic neuronal activity following PNN loss (30). Interestingly, evidence from postmortem studies suggest that a similar disruption of inhibitory activity may occur in subjects with schizophrenia in brain regions that have been reported to exhibit PNN abnormalities, including the amygdala, entorhinal cortex and prefrontal cortex (27-29). Along these lines, deficits in PNN structure within the prefrontal cortex may affect neuronal network oscillations in the gamma frequency band (30-80 Hz), which are mediated by parvalbumin-expressing neurons and have been shown to be involved in higher cognitive functions such as feature binding, attention, and working memory (92-94). The research findings showing that PNNs play a role in the regulation of glutamate receptor trafficking (89) could be of pathological significance in terms of the generation and maintenance of gamma band oscillations. In this regard, if confirmed at the translational level, the reduction of NMDA receptor mRNA expression in parvalbumin-expressing neurons reported in the prefrontal cortex of subjects with schizophrenia (95) may represent a potential pathological sequalae linked to PNN functional impairment. Notably, deficits in PNN function could affect the lateral diffusion of glutamate receptors within the plasma membrane, as well as receptor clustering within the synapse which coupled together may lead to deficits in synaptic regulation and plasticity (89). Direct testing of this hypothesis may be possible via fluorescence recovery after photobleaching using appropriate animal models.
Neuroprotection
Emerging evidence has shown PNNs to play a neuroprotective role in the CNS under oxidative stress, which may ensue from reduced levels of glutathione, an important antioxidant in the brain that has been reported to be decreased in schizophrenia patients (96). Components of the PNN, such as aggrecan, Tn-R, and link proteins may represent the basis for the neuroprotective status of these structures (97). However, PNNs may in turn be vulnerable to oxidative stress, as shown in mice with a genetic redox dysregulation (25). Against this background, a recent study by Morishita and colleagues has provided evidence of a prolonged period of brain plasticity in young adult mice under conditions of parvalbumin cell-specific glutathione dysregulation in vivo (98). Given that PNNs play a key role in balancing plasticity and stability of cortical circuits via parvalbumin cells (99), the authors of this study suggest that a redox-sensitive failure to maintain parvalbumin cells enwrapped by PNNs can result in mistimed developmental trajectories of brain plasticity which may contribute, in part, to the etiology of schizophrenia (100).
In keeping with the theme of oxidative stress, downstream effects of oxidative damage on neurons have been shown to involve specific gene promoters resulting in gene silencing (101). The mechanism of transcriptional regulation is likely epigenetic; specifically, it may be mediated through dysregulation of histone acetylation by histone deacetylase 2 (HDAC2). The chromatin modulating function of HDAC2 can be negatively regulated following a post-translational process called S-nitrosylation. Notably, HDAC2 nitrosylation has been shown to have a role in regulating both cortical development and memory formation (102, 103). Neuronal nitric oxide synthase, an enzyme that provides a major source of nitric oxide in neurons, is highly expressed specifically in the parvalbumin interneurons in the adult mouse cortex (104) and has been demonstrated to have positive effects on neuronal plasticity (105) as well as learning and memory (102). In this respect, nitric oxide has been linked to schizophrenia pathogenesis (100). An interesting hypothesis that therefore waits to be tested is whether negative regulation of HDAC2 by nitric oxide and its effect on transcription may modulate the activity of inhibitory circuits in a manner that prolongs windows of critical period plasticity resulting in long-term neurodevelopmental sequalae relevant to schizophrenia.
Development and Synaptic Plasticity
Given that PNNs are known to play a functional role in the regulation of developmental synaptic pruning in the cerebral cortex and that a synaptic pruning deficit has long been speculated to contribute to the onset of schizophrenia (12, 15), a deficit in the formation of PNNs could very well compromise the experience-dependent consolidation of synaptic connectivities, resulting in a protraction of the synaptic pruning process. In this regard, site-specific digestion of CSPGs in the hippocampus of animals using ChABC was shown to induce altered spine dynamics via a restriction of β1-integrin activation and signaling at synaptic sites (106). These findings are notable based on the evidence that dendritic spine density on pyramidal cells is decreased in schizophrenia (107-109). Interestingly, unpublished observations from our laboratory have shown that the proportion of pyramidal neurons surrounded by PNNs is decreased by 25-60% in layers III and V of the prefrontal cortex of subjects with schizophrenia. Of note are the neuropathological findings of decreased spine density and dendritic branching of these pyramidal neurons (107, 109-111).
The recent evidence showing that Semaphorin 3A (Sema3A) localizes to PNNs is of interest from a neurodevelopmental standpoint of schizophrenia. Sema3A is a potent regulator of neurite growth (112) and cell migration (113) in the developing nervous system, and it has strong effects on synapse dynamics (114). A disulfated chondroitin sulfate-E motif in the CSPGs is responsible for binding of Sema3A to PNNs (115). The observation that Sema3A is present on PNNs, coupled with the established role of PNNs in controlling plasticity, suggests that PNNs may exert their effect partly through Sema3A. Interestingly, evidence from both genetic and postmortem studies link Sema3A and its receptor family to schizophrenia. Notably, genome-wide association studies (GWAS) have identified variants within the semaphorin receptor component plexinA2 gene and the Sema3D gene to influence susceptibility to schizophrenia (116, 117). In a separate study, Eastwood and colleagues reported an increase of Sema3A mRNA expression in the cerebellum of schizophrenia subjects together with a decrease in the expression of synaptophysin and reelin, both of which are important for synaptic formation and maintenance, and have independently been linked to schizophrenia (118). In view of these observations future studies applying both in vitro and in vivo approaches to assess whether more of the molecules that guide axons during development bind to PNNs in the adult CNS and whether they have effects on synapse dynamics will be of key importance.
If we now turn our focus to non-cell-autonomous factors, specifically OTX2, several issues can be raised. A very general issue is the identification of the transfer mechanisms of this homeoprotein in the context of cerebral function. Although present in the extracellular space throughout the cortex, OTX2 is only internalized by parvalbumin interneurons after activity-dependent PNN assembly (75). Considering the role of OTX2 in maintaining the non-plastic state of the adult brain, it can be speculated that changes in the levels of this homeoprotein would interfere with downstream molecular pathways regulated by OTX2 within parvalbumin-expressing interneurons which could subsequently result in the brain retaining the “juvenile-like state” of plasticity characteristic of schizophrenia (14, 26, 71, 98). In order to answer this vital question, studies evaluating the main source of OTX2 using both appropriate animal models of schizophrenia and patients with schizophrenia will be crucial. In addition, it will also be important to evaluate the regional and developmental expression of OTX2 expression in both animals and humans in order to shed light as to when and where the changes in the expression of this homeoprotein occur during ontogeny. Moreover, investigation of the target genes and proteins of OTX2 will reveal further insights into the mechanisms linking experience, GABAergic circuit maturation, and critical period plasticity in the context of neurodevelopmental disorders such as schizophrenia. In this regard, a major endogenous source of OTX2 has been identified as the choroid plexus (74, 119, 120) that lines the ventricles, which incidentally have commonly been found to be enlarged in patients with schizophrenia (121).
Another interesting element that may be linked to schizophrenia pathophysiology is the biophysics of the ECM. Given that diffusion in the extracellular space is dependent on the structure and chemical properties of the ECM (122), it is conceivable that PNN abnormalities in the adult CNS may alter the efficacy of synapses and transmitter release associated with schizophrenia (123). Such changes could affect the efficacy of signal transmission at synapses by altering neuronal synchronization and neuron-glia communication but also by affecting synaptic and extrasynaptic volume transmission (122, 124, 125), notable examples include dopamine and glutamate (124, 125).
Finally, the Nogo receptor (NgR), which binds myelin-associated proteins (i.e., Nogo proteins) and has an inhibitory effect on neurite growth, is expressed in PNN-encapsulated neurons (126). Notably, this receptor has also been shown to bind CSPGs (127). Therefore, myelin formation in tandem with PNN structural integrity, may provide an additional mechanism that progressively restricts synaptic plasticity with a subsequent effect on stabilizing cortical circuitry in the mature brain. Myelin dysfunction may therefore further compromise the stability and integrity of synaptic connectivities. Interestingly, both Nogo and NgR have been linked to the pathogenesis of schizophrenia (128).
Potential Causes of PNN Abnormalities
The pathological mechanism(s) governing a PNN deficit in terms of the various schizophrenia hypotheses remains an open question (see Table 4). It is possible that this deficit could result from an alteration in proteinases involved in regulating the dynamic nature of the ECM. Two families of endogenous, extracellular metalloproteinases cleave ECM components: matrix metalloproteinase (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs). The cleavage of ECM components is part of their normal turnover process, but a dysregulation in this process may represent a prerequisite for neurological disease (129, 130). Interestingly, a recent GWAS has identified MMP-16 as a schizophrenia risk gene (131) and MMP-9 has also been implicated in this disorder (132). Towards this end, evidence from a recent study revealed that pyramidal neurons from layer III of the superior temporal gyrus of patients with schizophrenia exhibit alterations in the expression of many genes that encode both MMPs and ADAMTSs, including MMP16 (Table 2) (133). In a complementary study that used the same cohort of schizophrenia subjects, it was shown that genes which encode ADAMTS in addition to several key components of the PNN, including aggrecan, hyaluronan and laminin, were differentially expressed in parvalbumin-expressing interneurons (Table 3) (134). More recently, an extracellular increase in the level of clusterin (apolipoprotein-J) in the prefrontal cortex of patients with schizophrenia was reported (135). Although the pathological basis for this increase in clusterin remains unknown, given that enzymatic activity MMP-9 has been shown to be inhibited by clusterin (136), an increased expression of clusterin expression may represent a compensatory mechanism to promote the integrity of PNNs (135). Taken together, these observations suggest that a dysregulation in the remodeling of the ECM may represent a genuine component underlying the pathophysiology of the disease.
Table 4.
The possible link between PNN deficits and prominent schizophrenia hypotheses
| Neuropathological Hypothesis | Contributory effect of PNN |
|---|---|
| Dopamine | Unknown |
| Glutamate | Anomalies in trafficking of glutamatergic receptors |
| GABA | Impairment in the maturation of parvalbumin interneurons/inhibitory circuitry |
| Immune Dysregulation | Compromised PNNs mediated by MMPs |
| Myelination | Interaction with PNN via Nogo |
| Oxidative Stress | Compromised antioxidant defense system on the surface of neurons |
| Neurodevelopment | Temporal anomalies in the onset and closure of critical periods |
| Reduced Neuropil | Deficits in the stabilization and formation of synapses |
| Synaptic Connectivity | Deficits in the stabilization of synapses |
| Synaptic Prunning | Deficits in the formation of synapses |
Table 2.
Differentially expressed genes associated with extracellular matrix in pyramidal neurons in schizophrenia
| Gene Title | Gene Symbol | Direction of Change |
|---|---|---|
| Aggrecan | ACAN | Down |
| ADAM metallopeptidase with thrombospondin type 1 motif, 1 | ADAMTS1 | Up |
| ADAM metallopeptidase with thrombospondin type 1 motif, 1 | ADAMTS8 | Down |
| Hyaluronan and proteoglycan link protein 1 | HAPLN1 | Down |
| Leucine proline-enriched proteoglycan (leprecan) 1 | LEPRE1 | Down |
| Lumican | LUM | Down |
| Matrix metallopeptidase 16 (membrane-inserted) | MMP16 | Down |
| Matrix metallopeptidase 24 (membrane-inserted) | MMP24 | Up |
| Matrix metallopeptidase 25 | MMP25 | Down |
| Sperm adhesion molecule 1 | SPAM1 | Up |
| Sparc/osteonectin, cwcv and kazal-like domains proteoglycan 3 | SPOCK3 | Up |
| Spondin 1, extracellular matrix protein | SPON1 | Up |
| Versican | VCAN | Down |
* Reproduced from (23)
Table 3.
Differentially expressed genes associated with extracellular matrix in parvalbumin-containing neurons in schizophrenia
| Gene Title | Gene Symbol | Direction of Change |
|---|---|---|
| Aggrecan | ACAN | Up |
| ADAM metallopeptidase domain 7 | ADAM7 | Up |
| ADAM metallopeptidase with thrombospondin type 1 motif, 6 | ADAMTS6 | Up |
| Hyaluronan binding protein 4 | HABP4 | Up |
| Laminin, beta 1 | LAMB1 | Down |
Along these lines, an impressive study utilizing a single-cell reverse transcriptase multiplex polymerase chain reaction approach identified various clusters of parvalbumin cells that display distinct electrophysological profiles and exhibit different expression profiles in a subset of genes (137). Among these clusters were a subset of cells that expressed both parvalbumin and somatostatin and a subset of cells which expressed parvalbumin in conjunction with three metalloproteinases (Adamts8, Adamts15 and neprylsin). Notably, Wisteria floribunda agglutinin staining of PNNs revealed the subset of parvalbumin interneurons which expressed metalloproteinases to be surrounded by PNNs, as opposed to the parvalbumin interneurons that co-expressed somatostatin and interneurons that solely expressed somatostatin (137). The authors of this study suggest that somatostatin-expressing interneurons and the parvalbumin interneurons which co-express somatostatin are not protected by PNNs and may represent distinct classes of the interneurons that are highly susceptible to developmental insults, such as oxidative stress (137).
Microglia activation represents an intriguing pathological mechanism for PNN deficits observed in schizophrenia. In this respect, degradation of the PNN has been reported to be a feature of multiple sclerosis (138). The loss of PNNs in multiple sclerosis has been attributed to the production of MMP-9 by activated microglia (138) and, of interest, the upregulation of MMP-9 may be a key event that links oxidative stress to inflammation (139). In the same vein, the neuropathology of schizophrenia has been reported to be closely associated with cytokines and microglial activation (9). It is thus of note that the inflammatory response can provide a source of free radicals with the capacity to modify proteins, lipids, and nucleic acids (i.e., oxidative stress) that are potentially toxic for neurons and PNNs (140). Due to the positive feedback loops formed in such a mechanism, the disease state could self-sustain and persist, resulting in the progressive PNN deficits.
Conclusion and Perspectives
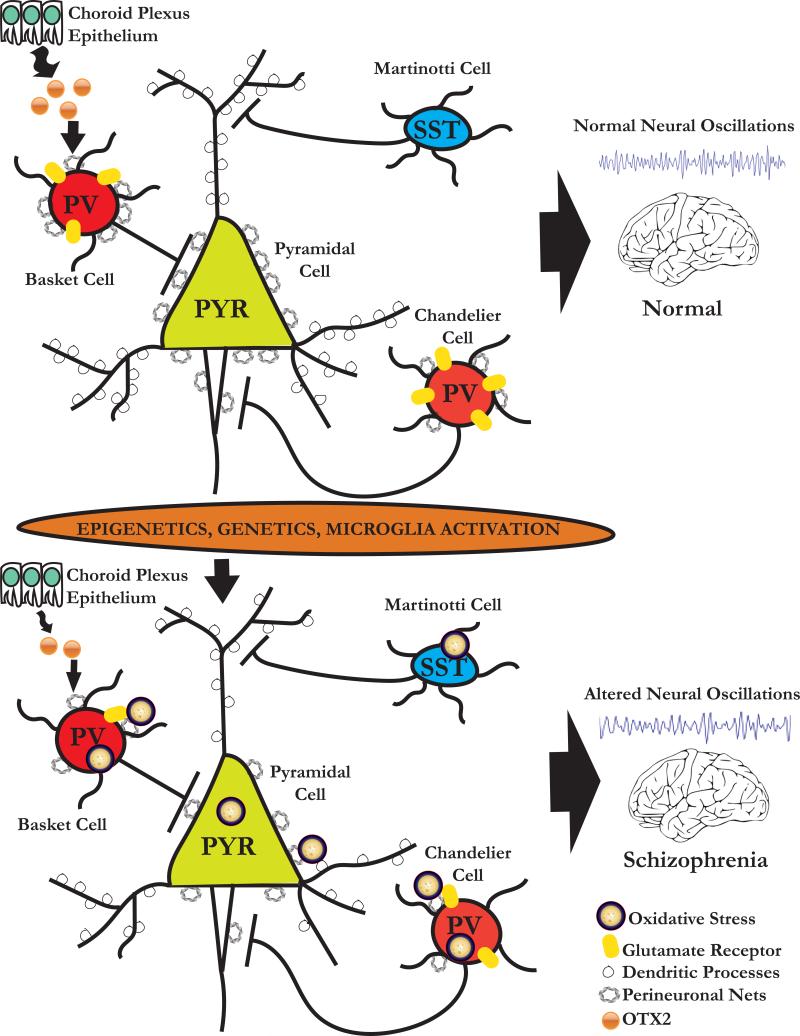
As the function of the PNN in the CNS continues to become unravelled, increasing evidence indicates that this structure not only plays an important role in neurophysiology and neuroplasticity but that PNN abnormalities can contribute to pathophysiological damage of the CNS, as observed in schizophrenia. The potential importance of the PNN in schizophrenia is highlighted by the neuropathological evidence demonstrating that this structure is altered in postmortem brain of these patients. The abnormalities of PNNs may stem from complex interactions of genetic vulnerabilities of genes encoding for CSPGs and metalloproteinases with environmental factors, which may subsequently interfere with postnatal neuronal maturation, protection from oxidative stress, synaptic regulation and plasticity of distinct interneuronal populations, potentially accounting for molecular and functional anomalies of neural circuitry (Figure 1) (24). Together, these pathological sequalae may culminate in the disruption of cognitive and emotional processing associated with schizophrenia.
Figure 1.
Schematic diagram of the potential neurobiological mechanisms associated with circuitry dysfunction in schizophrenia. During normal postnatal development, progressive increase in inhibitory inputs to pyramidal (PYR) neurons furnished by parvalbumin (PV) and somatostatin (SST) interneurons enables PYR neuronal circuits to oscillate in gamma and theta band frequencies, respectively. Epigenetic and genetic susceptibility in addition to microglia activation can provide a source of free radicals with the capacity to modify proteins, lipids, and nucleic acids (i.e., oxidative stress) that reduce N-Methyl-d-Aspartate (NMDA) receptor activity and which are potentially toxic for neurons and perineuronal nets (PNNs). The reduction in PNNs results in a deficit of OTX2 internalization into PV interneurons. As a consequence, PV-bearing PNNs are impaired, as manifested by alterations of local oscillations and distant synchronization. Because PNNs are protective of neurons from oxidative stress, PNN deficits may render them more vulnerable to oxidative injury. These cellular and molecular changes may alter the timing of critical periods.
Gaps in our knowledge remain as to whether PNNs serve similar functions for inhibitory and excitatory cells or subsets of these cells. Future studies aimed at identifying the functions and molecular and physiological parameters that differentiate the cells that have nets from those that do not will therefore be of key importance. In this context, it will be essential to identify and examine which CSPG members or other classes of ECM molecules are directly affected in schizophrenia and whether they are equally affected in different subset of interneurons and pyramidal cells. With this in mind, the use of gene set enrichment analysis to evaluate PNN genes and PNN interacting genes in the context of key genetic and environmental aspects that have been linked to schizophrenia through appropriate animal models, will prove integral in terms of elucidating if a clear biological distinction exists in terms of the PNN deficits observed in schizophrenia.
There remain significant challenges to rescuing the aberrant connectional and synaptic assembly associated with schizophrenia. Nonetheless, it could be speculated that therapeutic strategies which modulate the maturation and circuit integration of parvalbumin interneurons and PNNs via factors such as OTX2 (72, 74, 98), brain-derived neurotrophic factor (141), glial-derived neurotropic factor (142), neuregulin-1 (143) and neuronal pentraxins (144) may offer a fresh therapeutic landscape for cognitive interventions targeting brain plasticity in schizophrenia.
Acknowledgements
This work was supported by Grants MH076060 and MH080272 from the National Institutes of Health (T-U.W.W). We are grateful to all brain donors and their families for tissue samples included in the studies described. We would like to express our thanks to Dr. Clemente Garcia-Rizo, M.D., Ph.D., Dr. Brice Mouttet, M.D. and Victor Kouassi, M.Phil. for reading and providing insightful feedback on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors of this manuscript report no biomedical financial interests or potential conflict of interest to disclose.
References
- 1.Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987;295:681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 3.Keshavan MS, Giedd J, Lau JY, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1:549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- 4.Bakhshi K, Chance SA. The neuropathology of schizophrenia: A selective review of past studies and emerging themes in brain structure and cytoarchitecture. Neuroscience. 2015;303:82–102. doi: 10.1016/j.neuroscience.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Iyegbe C, Campbell D, Butler A, Ajnakina O, Sham P. The emerging molecular architecture of schizophrenia, polygenic risk scores and the clinical implications for GxE research. Soc Psychiatry Psychiatr Epidemiol. 2014;49:169–182. doi: 10.1007/s00127-014-0823-2. [DOI] [PubMed] [Google Scholar]
- 6.van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volk DW, Lewis DA. The Role of Endocannabinoid Signaling in Cortical Inhibitory Neuron Dysfunction in Schizophrenia. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Forti M, Marconi A, Carra E, Fraietta S, Trotta A, Bonomo M, et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry. 2015;2:233–238. doi: 10.1016/S2215-0366(14)00117-5. [DOI] [PubMed] [Google Scholar]
- 9.Girgis RR, Kumar SS, Brown AS. The cytokine model of schizophrenia: emerging therapeutic strategies. Biol Psychiatry. 2014;75:292–299. doi: 10.1016/j.biopsych.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- 11.van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull. 2008;34:1095–1105. doi: 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 13.Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- 14.Woo TU. Neurobiology of schizophrenia onset. Curr Top Behav Neurosci. 2014;16:267–295. doi: 10.1007/7854_2013_243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 16.Anticevic A, Cole MW, Repovs G, Savic A, Driesen NR, Yang G, et al. Connectivity, pharmacology, and computation: toward a mechanistic understanding of neural system dysfunction in schizophrenia. Front Psychiatry. 2013;4:169. doi: 10.3389/fpsyt.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol. 2012:267–295. doi: 10.1007/978-3-642-25758-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardingham GE, Do KQ. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci. 2016;17:125–134. doi: 10.1038/nrn.2015.19. [DOI] [PubMed] [Google Scholar]
- 19.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 20.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 21.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 22.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bitanihirwe BK, Woo TU. Perineuronal nets and schizophrenia: The importance of neuronal coatings. Neurosci Biobehav Rev. 2014;45:85–99. doi: 10.1016/j.neubiorev.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET. Losing the sugar coating: Potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res. 2015;167:18–27. doi: 10.1016/j.schres.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-Life Insults Impair Parvalbumin Interneurons via Oxidative Stress: Reversal by N-Acetylcysteine. Biol Psychiatry. 2013;73:574–582. doi: 10.1016/j.biopsych.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Do KQ, Cuenod M, Hensch TK. Targeting Oxidative Stress and Aberrant Critical Period Plasticity in the Developmental Trajectory to Schizophrenia. Schizophr Bull. 2015;41:835–846. doi: 10.1093/schbul/sbv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74:427–435. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–166. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pantazopoulos H, Markota M, Jaquet F, Ghosh D, Wallin A, Santos A, et al. Aggrecan and chondroitin-6-sulfate abnormalities in schizophrenia and bipolar disorder: a postmortem study on the amygdala. Translational psychiatry. 2015;5:e496. doi: 10.1038/tp.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah A, Lodge DJ. A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Translational psychiatry. 2013;3:e215. doi: 10.1038/tp.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steullet P, Cabungcal JH, Monin A, Dwir D, O'Donnell P, Cuenod M, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A “central hub” in schizophrenia pathophysiology? Schizophr Res. 2014 doi: 10.1016/j.schres.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349:147–160. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- 33.Frischknecht R, Gundelfinger ED. The brain's extracellular matrix and its role in synaptic plasticity. Advances in experimental medicine and biology. 2012;970:153–171. doi: 10.1007/978-3-7091-0932-8_7. [DOI] [PubMed] [Google Scholar]
- 34.Tsien RY. Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc Natl Acad Sci U S A. 2013;110:12456–12461. doi: 10.1073/pnas.1310158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 36.Welham J, Isohanni M, Jones P, McGrath J. The antecedents of schizophrenia: a review of birth cohort studies. Schizophr Bull. 2009;35:603–623. doi: 10.1093/schbul/sbn084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 38.Koppe G, Bruckner G, Brauer K, Hartig W, Bigl V. Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res. 1997;288:33–41. doi: 10.1007/s004410050790. [DOI] [PubMed] [Google Scholar]
- 39.Giamanco KA, Morawski M, Matthews RT. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience. 2010;170:1314–1327. doi: 10.1016/j.neuroscience.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 40.Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71:1073–1089. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- 41.Bruckner G, Grosche J, Schmidt S, Hartig W, Margolis RU, Delpech B, et al. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol. 2000;428:616–629. doi: 10.1002/1096-9861(20001225)428:4<616::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 42.Dauth S, Grevesse T, Pantazopoulos H, Campbell PH, Maoz BM, Berretta S, et al. Extracellular matrix protein expression is brain region dependent. J Comp Neurol. 2016 doi: 10.1002/cne.23965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilbig H, Nowack S, Boeckler K, Bidmon HJ, Zilles K. Characterization of neuronal subsets surrounded by perineuronal nets in the rhesus auditory brainstem. J Anat. 2007;210:507–517. doi: 10.1111/j.1469-7580.2007.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartig W, Bruckner G, Brauer K, Schmidt C, Bigl V. Allocation of perineuronal nets and parvalbumin-, calbindin-D28k- and glutamic acid decarboxylase-immunoreactivity in the amygdala of the rhesus monkey. Brain Res. 1995;698:265–269. doi: 10.1016/0006-8993(95)01016-o. [DOI] [PubMed] [Google Scholar]
- 45.Hartig W, Klein C, Brauer K, Schuppel KF, Arendt T, Bigl V, et al. Hyperphosphorylated protein tau is restricted to neurons devoid of perineuronal nets in the cortex of aged bison. Neurobiol Aging. 2001;22:25–33. doi: 10.1016/s0197-4580(00)00179-2. [DOI] [PubMed] [Google Scholar]
- 46.Atoji Y, Yamamoto Y, Suzuki Y, Matsui F, Oohira A. Immunohistochemical localization of neurocan in the lower auditory nuclei of the dog. Hear Res. 1997;110:200–208. doi: 10.1016/s0378-5955(97)00079-8. [DOI] [PubMed] [Google Scholar]
- 47.Cant NB, Benson CG. Wisteria floribunda lectin is associated with specific cell types in the ventral cochlear nucleus of the gerbil, Meriones unguiculatus. Hear Res. 2006;216-217:64–72. doi: 10.1016/j.heares.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Foster NL, Mellott JG, Schofield BR. Perineuronal nets and GABAergic cells in the inferior colliculus of guinea pigs. Front Neuroanat. 2014;7:53. doi: 10.3389/fnana.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balmer TS, Carels VM, Frisch JL, Nick TA. Modulation of perineuronal nets and parvalbumin with developmental song learning. J Neurosci. 2009;29:12878–12885. doi: 10.1523/JNEUROSCI.2974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H, He D, Lasek AW. Repeated Binge Drinking Increases Perineuronal Nets in the Insular Cortex. Alcohol Clin Exp Res. 2015;39:1930–1938. doi: 10.1111/acer.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 52.Lee H, Leamey CA, Sawatari A. Perineuronal nets play a role in regulating striatal function in the mouse. PLoS One. 2012;7:e32747. doi: 10.1371/journal.pone.0032747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coleman LG, Jr., Liu W, Oguz I, Styner M, Crews FT. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav. 2014;116:142–151. doi: 10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romberg C, Yang S, Melani R, Andrews MR, Horner AE, Spillantini MG, et al. Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J Neurosci. 2013;33:7057–7065. doi: 10.1523/JNEUROSCI.6267-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue YX, Xue LF, Liu JF, He J, Deng JH, Sun SC, et al. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J Neurosci. 2014;34:6647–6658. doi: 10.1523/JNEUROSCI.5390-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohira K, Takeuchi R, Iwanaga T, Miyakawa T. Chronic fluoxetine treatment reduces parvalbumin expression and perineuronal nets in gamma-aminobutyric acidergic interneurons of the frontal cortex in adult mice. Molecular brain. 2013;6:43. doi: 10.1186/1756-6606-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pantazopoulos H, Murray EA, Berretta S. Total number, distribution, and phenotype of cells expressing chondroitin sulfate proteoglycans in the normal human amygdala. Brain Res. 2008;1207:84–95. doi: 10.1016/j.brainres.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lendvai D, Morawski M, Negyessy L, Gati G, Jager C, Baksa G, et al. Neurochemical mapping of the human hippocampus reveals perisynaptic matrix around functional synapses in Alzheimer's disease. Acta Neuropathol. 2013;125:215–229. doi: 10.1007/s00401-012-1042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hausen D, Bruckner G, Drlicek M, Hartig W, Brauer K, Bigl V. Pyramidal cells ensheathed by perineuronal nets in human motor and somatosensory cortex. Neuroreport. 1996;7:1725–1729. doi: 10.1097/00001756-199607290-00006. [DOI] [PubMed] [Google Scholar]
- 60.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 61.Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15:414–422. S411–412. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- 62.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krishnan K, Wang BS, Lu J, Wang L, Maffei A, Cang J, et al. MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proc Natl Acad Sci U S A. 2015;112:E4782–4791. doi: 10.1073/pnas.1506499112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016 doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guimaraes A, Zaremba S, Hockfield S. Molecular and morphological changes in the cat lateral geniculate nucleus and visual cortex induced by visual deprivation are revealed by monoclonal antibodies Cat-304 and Cat-301. J Neurosci. 1990;10:3014–3024. doi: 10.1523/JNEUROSCI.10-09-03014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hockfield S, Kalb RG, Zaremba S, Fryer H. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harb Symp Quant Biol. 1990;55:505–514. doi: 10.1101/sqb.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- 67.McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci. 2007;27:5405–5413. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faralli A, Dagna F, Albera A, Bekku Y, Oohashi T, Albera R, et al. Modifications of perineuronal nets and remodelling of excitatory and inhibitory afferents during vestibular compensation in the adult mouse. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-1095-7. [DOI] [PubMed] [Google Scholar]
- 69.Yamada J, Jinno S. Subclass-specific formation of perineuronal nets around parvalbumin-expressing GABAergic neurons in Ammon's horn of the mouse hippocampus. J Comp Neurol. 2015;523:790–804. doi: 10.1002/cne.23712. [DOI] [PubMed] [Google Scholar]
- 70.Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 71.Beurdeley M, Spatazza J, Lee HH, Sugiyama S, Bernard C, Di Nardo AA, et al. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci. 2012;32:9429–9437. doi: 10.1523/JNEUROSCI.0394-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prochiantz A, Di Nardo AA. Homeoprotein signaling in the developing and adult nervous system. Neuron. 2015;85:911–925. doi: 10.1016/j.neuron.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cardin AD, Weintraub HJ. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 74.Spatazza J, Lee HH, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK, et al. Choroidplexus-derived otx2 homeoprotein constrains adult cortical plasticity. Cell reports. 2013;3:1815–1823. doi: 10.1016/j.celrep.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, et al. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 76.Pantazopoulos H, Boyer-Boiteau A, Holbrook EH, Jang W, Hahn CG, Arnold SE, et al. Proteoglycan abnormalities in olfactory epithelium tissue from subjects diagnosed with schizophrenia. Schizophr Res. 2013;150:366–372. doi: 10.1016/j.schres.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11:735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- 78.Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J, et al. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J Neurosci. 2015;35:4190–4202. doi: 10.1523/JNEUROSCI.3592-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saghatelyan AK, Gorissen S, Albert M, Hertlein B, Schachner M, Dityatev A. The extracellular matrix molecule tenascin-R and its HNK-1 carbohydrate modulate perisomatic inhibition and long-term potentiation in the CA1 region of the hippocampus. Eur J Neurosci. 2000;12:3331–3342. doi: 10.1046/j.1460-9568.2000.00216.x. [DOI] [PubMed] [Google Scholar]
- 80.Bukalo O, Schachner M, Dityatev A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience. 2001;104:359–369. doi: 10.1016/s0306-4522(01)00082-3. [DOI] [PubMed] [Google Scholar]
- 81.Zaremba S, Guimaraes A, Kalb RG, Hockfield S. Characterization of an activity-dependent, neuronal surface proteoglycan identified with monoclonal antibody Cat-301. Neuron. 1989;2:1207–1219. doi: 10.1016/0896-6273(89)90305-x. [DOI] [PubMed] [Google Scholar]
- 82.Bruckner G, Morawski M, Arendt T. Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience. 2008;151:489–504. doi: 10.1016/j.neuroscience.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 83.Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K, et al. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–577. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- 84.Bertolotto A, Manzardo E, Guglielmone R. Immunohistochemical mapping of perineuronal nets containing chondroitin unsulfated proteoglycan in the rat central nervous system. Cell Tissue Res. 1996;283:283–295. doi: 10.1007/s004410050538. [DOI] [PubMed] [Google Scholar]
- 85.Sonntag M, Blosa M, Schmidt S, Rubsamen R, Morawski M. Perineuronal nets in the auditory system. Hear Res. 2015;329:21–32. doi: 10.1016/j.heares.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 86.Jager C, Lendvai D, Seeger G, Bruckner G, Matthews RT, Arendt T, et al. Perineuronal and perisynaptic extracellular matrix in the human spinal cord. Neuroscience. 2013;238:168–184. doi: 10.1016/j.neuroscience.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 87.Happel MF, Niekisch H, Castiblanco Rivera LL, Ohl FW, Deliano M, Frischknecht R. Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proc Natl Acad Sci U S A. 2014;111:2800–2805. doi: 10.1073/pnas.1310272111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- 89.Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 90.Heine M, Thoumine O, Mondin M, Tessier B, Giannone G, Choquet D. Activity-independent and subunit-specific recruitment of functional AMPA receptors at neurexin/neuroligin contacts. Proc Natl Acad Sci U S A. 2008;105:20947–20952. doi: 10.1073/pnas.0804007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, et al. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci U S A. 2015;112:3535–3540. doi: 10.1073/pnas.1413625112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woo TU, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18:173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bitanihirwe BK, Lim MP, Kelley JF, Kaneko T, Woo TU. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 97.Suttkus A, Rohn S, Weigel S, Glockner P, Arendt T, Morawski M. Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell death & disease. 2014;5:e1119. doi: 10.1038/cddis.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morishita H, Cabungcal JH, Chen Y, Do KQ, Hensch TK. Prolonged Period of Cortical Plasticity upon Redox Dysregulation in Fast-Spiking Interneurons. Biol Psychiatry. 2015;78:396–402. doi: 10.1016/j.biopsych.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501:543–546. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bitanihirwe BK, Woo TU. Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev. 2011;35:878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 102.Graff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J, et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell. 2014;156:261–276. doi: 10.1016/j.cell.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 104.Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, et al. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex. 2011;21:1803–1817. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- 105.Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011;14:2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orlando C, Ster J, Gerber U, Fawcett JW, Raineteau O. Perisynaptic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J Neurosci. 2012;32:18009–18017. doi: 10.1523/JNEUROSCI.2406-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 108.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- 111.Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA psychiatry. 2014;71:1323–1331. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schwarting GA, Kostek C, Ahmad N, Dibble C, Pays L, Puschel AW. Semaphorin 3A is required for guidance of olfactory axons in mice. J Neurosci. 2000;20:7691–7697. doi: 10.1523/JNEUROSCI.20-20-07691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen G, Sima J, Jin M, Wang KY, Xue XJ, Zheng W, et al. Semaphorin-3A guides radial migration of cortical neurons during development. Nat Neurosci. 2008;11:36–44. doi: 10.1038/nn2018. [DOI] [PubMed] [Google Scholar]
- 114.Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, et al. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462:1065–1069. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dick G, Tan CL, Alves JN, Ehlert EM, Miller GM, Hsieh-Wilson LC, et al. Semaphorin 3A binds to the perineuronal nets via chondroitin sulfate type E motifs in rodent brains. J Biol Chem. 2013;288:27384–27395. doi: 10.1074/jbc.M111.310029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mah S, Nelson MR, Delisi LE, Reneland RH, Markward N, James MR, et al. Identification of the semaphorin receptor PLXNA2 as a candidate for susceptibility to schizophrenia. Mol Psychiatry. 2006;11:471–478. doi: 10.1038/sj.mp.4001785. [DOI] [PubMed] [Google Scholar]
- 117.Fujii T, Uchiyama H, Yamamoto N, Hori H, Tatsumi M, Ishikawa M, et al. Possible association of the semaphorin 3D gene (SEMA3D) with schizophrenia. J Psychiatr Res. 2011;45:47–53. doi: 10.1016/j.jpsychires.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 118.Eastwood SL, Law AJ, Everall IP, Harrison PJ. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;8:148–155. doi: 10.1038/sj.mp.4001233. [DOI] [PubMed] [Google Scholar]
- 119.Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci. 2015;16:445–457. doi: 10.1038/nrn3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liddelow SA. Development of the choroid plexus and blood-CSF barrier. Front Neurosci. 2015;9:32. doi: 10.3389/fnins.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weinberger DR, Torrey EF, Neophytides AN, Wyatt RJ. Lateral cerebral ventricular enlargement in chronic schizophrenia. Arch Gen Psychiatry. 1979;36:735–739. doi: 10.1001/archpsyc.1979.01780070013001. [DOI] [PubMed] [Google Scholar]
- 122.Sykova E, Vorisek I, Mazel T, Antonova T, Schachner M. Reduced extracellular space in the brain of tenascin-R- and HNK-1-sulphotransferase deficient mice. Eur J Neurosci. 2005;22:1873–1880. doi: 10.1111/j.1460-9568.2005.04375.x. [DOI] [PubMed] [Google Scholar]
- 123.Earls LR, Zakharenko SS. A Synaptic Function Approach to Investigating Complex Psychiatric Diseases. Neuroscientist. 2013;20:257–271. doi: 10.1177/1073858413498307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De-Miguel FF, Fuxe K. Extrasynaptic neurotransmission as a way of modulating neuronal functions. Front Physiol. 2012;3:16. doi: 10.3389/fphys.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Zhang WB, Agnati LF. Volume transmission and its different forms in the central nervous system. Chin J Integr Med. 2013;19:323–329. doi: 10.1007/s11655-013-1455-1. [DOI] [PubMed] [Google Scholar]
- 126.Ye Q, Miao QL. Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol. 2013;32:352–363. doi: 10.1016/j.matbio.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 127.Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Willi R, Schwab ME. Nogo and Nogo receptor: relevance to schizophrenia? Neurobiol Dis. 2013;54:150–157. doi: 10.1016/j.nbd.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 129.Gottschall PE, Howell MD. ADAMTS expression and function in central nervous system injury and disorders. Matrix Biol. 44. 2015;46:70–76. doi: 10.1016/j.matbio.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–757. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ripke S, O'Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–1159. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lepeta K, Kaczmarek L. Matrix Metalloproteinase-9 as a Novel Player in Synaptic Plasticity and Schizophrenia. Schizophr Bull. 2015;41:1003–1009. doi: 10.1093/schbul/sbv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pietersen CY, Mauney SA, Kim SS, Lim MP, Rooney RJ, Goldstein JM, et al. Molecular profiles of pyramidal neurons in the superior temporal cortex in schizophrenia. Journal of neurogenetics. 2014;28:53–69. doi: 10.3109/01677063.2014.882918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pietersen CY, Mauney SA, Kim SS, Passeri E, Lim MP, Rooney RJ, et al. Molecular profiles of parvalbumin-immunoreactive neurons in the superior temporal cortex in schizophrenia. Journal of neurogenetics. 2014;28:70–85. doi: 10.3109/01677063.2013.878339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Athanas KM, Mauney SL, Woo TW. Increased extracellular clusterin in the prefrontal cortex in schizophrenia. Schizophr Res. 2015 doi: 10.1016/j.schres.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jeong S, Ledee DR, Gordon GM, Itakura T, Patel N, Martin A, et al. Interaction of clusterin and matrix metalloproteinase-9 and its implication for epithelial homeostasis and inflammation. Am J Pathol. 2012;180:2028–2039. doi: 10.1016/j.ajpath.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rossier J, Bernard A, Cabungcal JH, Perrenoud Q, Savoye A, Gallopin T, et al. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol Psychiatry. 2015;20:154–161. doi: 10.1038/mp.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gray E, Thomas TL, Betmouni S, Scolding N, Love S. Elevated matrix metalloproteinase-9 and degradation of perineuronal nets in cerebrocortical multiple sclerosis plaques. J Neuropathol Exp Neurol. 2008;67:888–899. doi: 10.1097/NEN.0b013e318183d003. [DOI] [PubMed] [Google Scholar]
- 139.Do K, Dwir D, Cabungcal JH, Tirouvanziam R, Cuenod M. Receptor for advanced glycation end-product (rage) as linking mechanism between neuroinflammation and oxidative stress. Schizophr Bull. 2015;41:S6. [Google Scholar]
- 140.Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–277. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 141.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 142.Canty AJ, Dietze J, Harvey M, Enomoto H, Milbrandt J, Ibanez CF. Regionalized loss of parvalbumin interneurons in the cerebral cortex of mice with deficits in GFRalpha1 signaling. J Neurosci. 2009;29:10695–10705. doi: 10.1523/JNEUROSCI.2658-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tamura H, Kawata M, Hamaguchi S, Ishikawa Y, Shiosaka S. Processing of neuregulin-1 by neuropsin regulates GABAergic neuron to control neural plasticity of the mouse hippocampus. J Neurosci. 2012;32:12657–12672. doi: 10.1523/JNEUROSCI.2542-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron. 2015;85:1257–1272. doi: 10.1016/j.neuron.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bruckner G, Bringmann A, Koppe G, Hartig W, Brauer K. In vivo and in vitro labelling of perineuronal nets in rat brain. Brain Res. 1996;720:84–92. doi: 10.1016/0006-8993(96)00152-7. [DOI] [PubMed] [Google Scholar]
- 146.Hartig W, Derouiche A, Welt K, Brauer K, Grosche J, Mader M, et al. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999;842:15–29. doi: 10.1016/s0006-8993(99)01784-9. [DOI] [PubMed] [Google Scholar]
- 147.Morawski M, Reinert T, Meyer-Klaucke W, Wagner FE, Troger W, Reinert A, et al. Ion exchanger in the brain: Quantitative analysis of perineuronally fixed anionic binding sites suggests diffusion barriers with ion sorting properties. Sci Rep. 2015;5:16471. doi: 10.1038/srep16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morawski M, Bruckner G, Jager C, Seeger G, Matthews RT, Arendt T. Involvement of perineuronal and perisynaptic extracellular matrix in Alzheimer's disease neuropathology. Brain Pathol. 2012;22:547–561. doi: 10.1111/j.1750-3639.2011.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110:9130–9135. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012;13:478–490. doi: 10.1038/nrn3258. [DOI] [PubMed] [Google Scholar]