Abstract
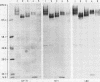
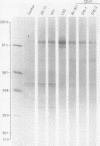
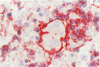
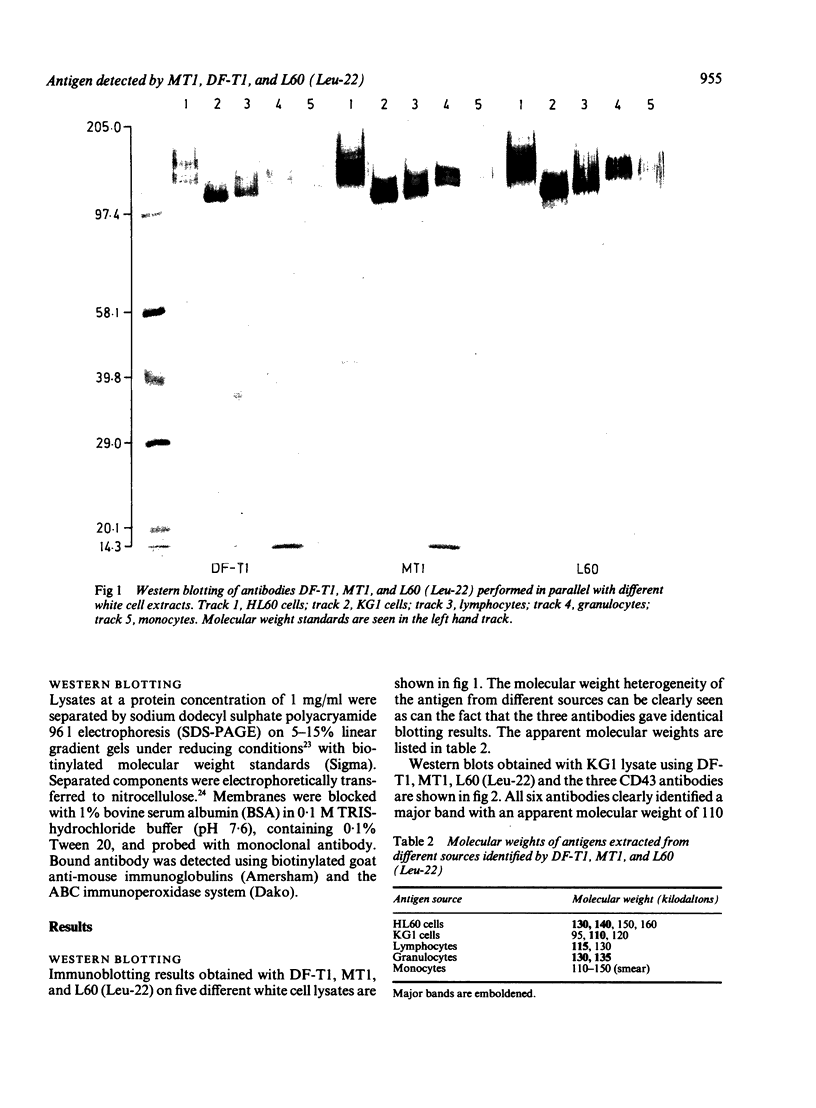
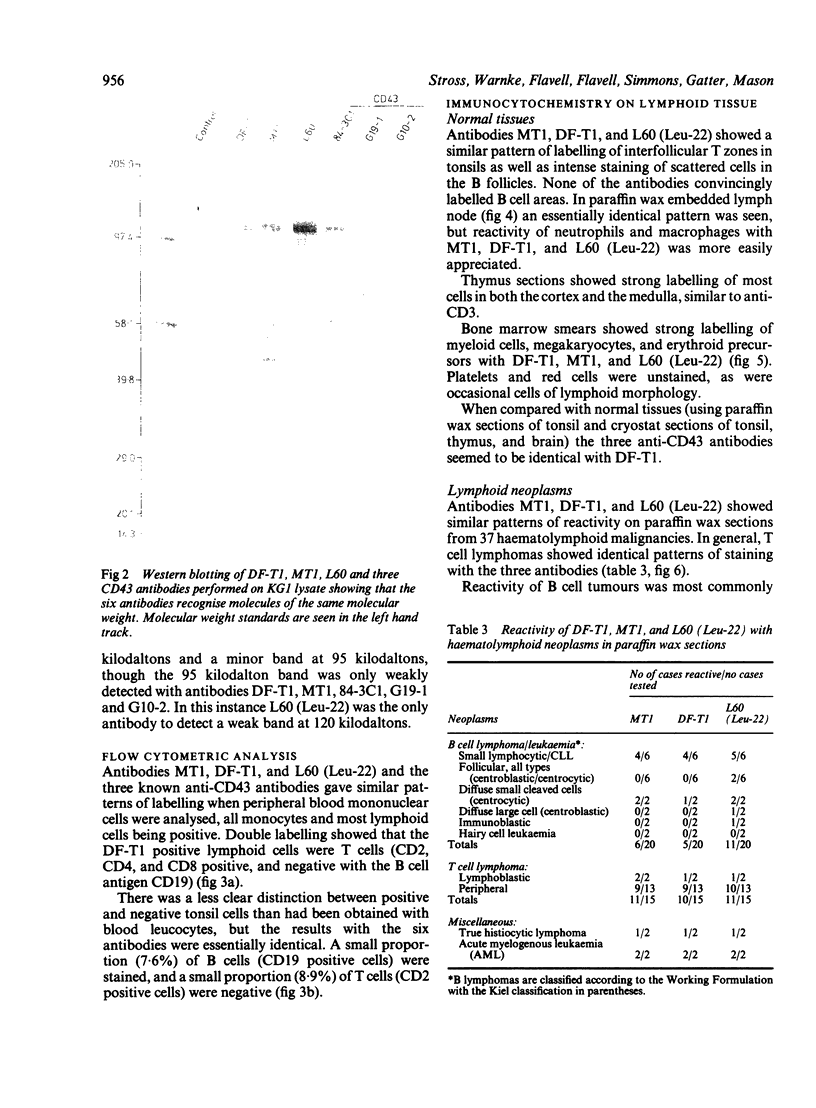
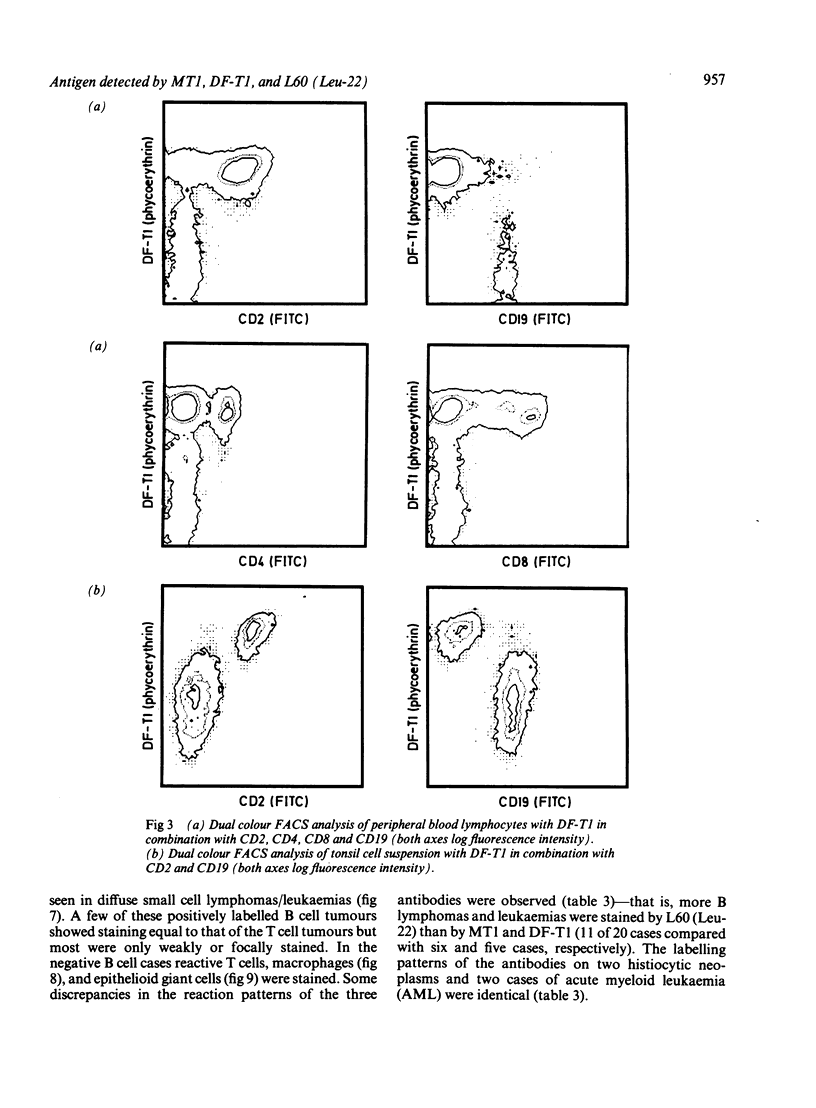
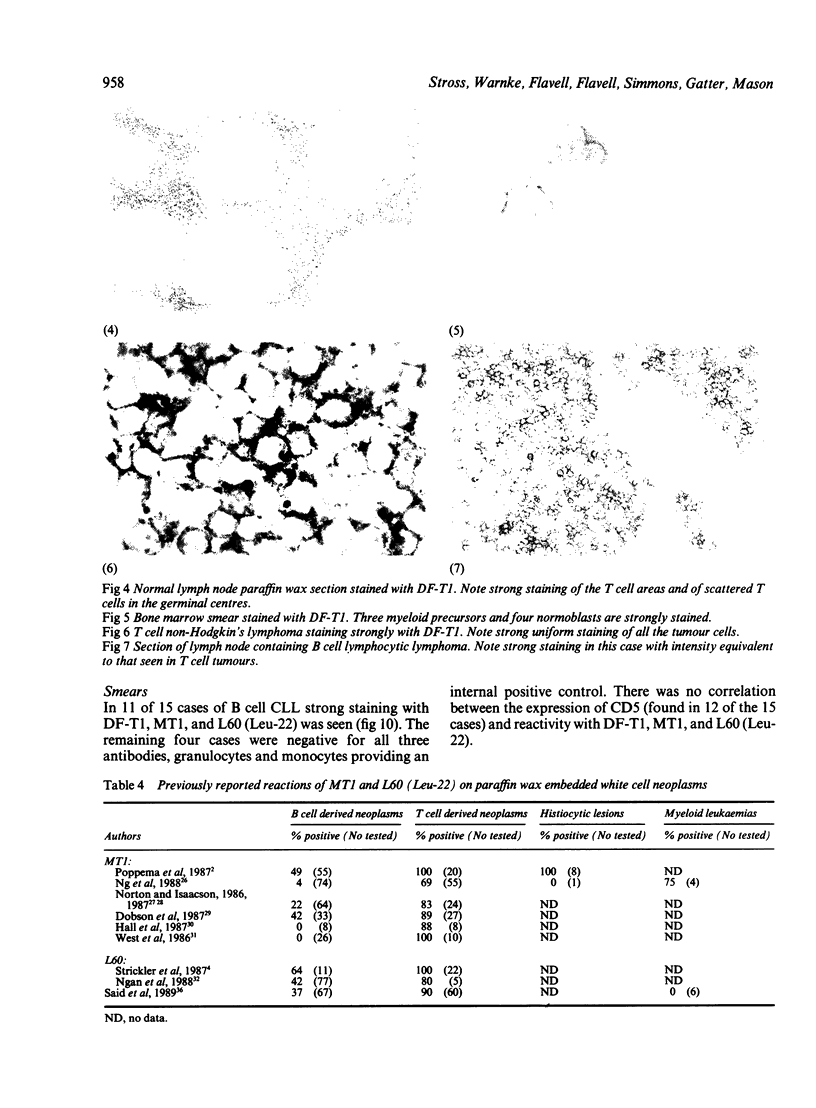
Three monoclonal antibodies MT1, L60 (Leu-22), and DF-T1, were reported independently as recognising human T cells in routinely processed, paraffin wax embedded tissue. The present study was performed to compare these three reagents in terms of their immunocytochemical reactions and target molecule(s). On Western blotting of white cell extracts the three antibodies reacted with antigens of the same molecular weight (range 110-160 kilodaltons). Furthermore, their immunocytochemical reactivity with normal human cells, as analysed by two-colour flow cytometry, was essentially identical (labelling of monocytes, most T lymphocytes, and weak reactions with some B cells), and the antibodies gave closely similar reactions on 54 white cell derived neoplasms. To identify the target antigen for these three reagents, antibodies from the Third International Workshop on Leucocyte Antigens were reviewed and it was shown that the Western blotting and immunocytochemical reactions of MT1, L60 (Leu-22), and DF-T1 were identical with those of the reagents which defined the CD43 antigen (also known as leucosialin or sialophorin). Furthermore, all these antibodies reacted with cells transfected with a cDNA clone encoding CD43. It is concluded that antibodies MT1, L60 (Leu-22), and DF-T1 all recognise the heavily glycosylated myeloid/lymphoid associated CD43 antigen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson B., Youseffi-Etemad R., Hammarström S., Perlmann P. Induction of aggregation and enhancement of proliferation and IL-2 secretion in human T cells by antibodies to CD43. J Immunol. 1988 Nov 1;141(9):2912–2917. [PubMed] [Google Scholar]
- Borche L., Lozano F., Vilella R., Vives J. CD43 monoclonal antibodies recognize the large sialoglycoprotein of human leukocytes. Eur J Immunol. 1987 Oct;17(10):1523–1526. doi: 10.1002/eji.1830171023. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Dobson C. M., Myskow M. W., Krajewski A. S., Carpenter F. H., Horne C. H. Immunohistochemical staining of non-Hodgkin's lymphoma in paraffin sections using the MB1 and MT1 monoclonal antibodies. J Pathol. 1987 Nov;153(3):203–212. doi: 10.1002/path.1711530304. [DOI] [PubMed] [Google Scholar]
- Facchetti F., De Wolf-Peeters C., van den Oord J. J., De vos R., Desmet V. J. Plasmacytoid T cells: a cell population normally present in the reactive lymph node. An immunohistochemical and electronmicroscopic study. Hum Pathol. 1988 Sep;19(9):1085–1092. doi: 10.1016/s0046-8177(88)80091-1. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Hall P. A., D'Ardenne A. J., Butler M. G., Habeshaw J. R., Stansfeld A. G. New marker of B lymphocytes, MB2: comparison with other lymphocyte subset markers active in conventionally processed tissue sections. J Clin Pathol. 1987 Feb;40(2):151–156. doi: 10.1136/jcp.40.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollema H., Poppema S. Immunophenotypes of malignant lymphoma centroblastic-centrocytic and malignant lymphoma centrocytic: an immunohistologic study indicating a derivation from different stages of B cell differentiation. Hum Pathol. 1988 Sep;19(9):1053–1059. doi: 10.1016/s0046-8177(88)80086-8. [DOI] [PubMed] [Google Scholar]
- Killeen N., Barclay A. N., Willis A. C., Williams A. F. The sequence of rat leukosialin (W3/13 antigen) reveals a molecule with O-linked glycosylation of one third of its extracellular amino acids. EMBO J. 1987 Dec 20;6(13):4029–4034. doi: 10.1002/j.1460-2075.1987.tb02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Gatter K. C. The role of immunocytochemistry in diagnostic pathology. J Clin Pathol. 1987 Sep;40(9):1042–1054. doi: 10.1136/jcp.40.9.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. Y., Krissansen G. W., Davey F. R., Crumpton M. J., Gatter K. C. Antisera against epitopes resistant to denaturation on T3 (CD3) antigen can detect reactive and neoplastic T cells in paraffin embedded tissue biopsy specimens. J Clin Pathol. 1988 Feb;41(2):121–127. doi: 10.1136/jcp.41.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzer S. J., Remold-O'Donnell E., Crimmins M. A., Bierer B. E., Rosen F. S., Burakoff S. J. Sialophorin, a surface sialoglycoprotein defective in the Wiskott-Aldrich syndrome, is involved in human T lymphocyte proliferation. J Exp Med. 1987 May 1;165(5):1383–1392. doi: 10.1084/jem.165.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C. S., Chan J. K., Hui P. K., Lo S. T. Monoclonal antibodies reactive with normal and neoplastic T cells in paraffin sections. Hum Pathol. 1988 Mar;19(3):295–303. doi: 10.1016/s0046-8177(88)80522-7. [DOI] [PubMed] [Google Scholar]
- Ngan B. Y., Picker L. J., Medeiros L. J., Warnke R. A. Immunophenotypic diagnosis of non-Hodgkin's lymphoma in paraffin sections. Co-expression of L60 (Leu-22) and L26 antigens correlates with malignant histologic findings. Am J Clin Pathol. 1989 May;91(5):579–583. doi: 10.1093/ajcp/91.5.579. [DOI] [PubMed] [Google Scholar]
- Norton A. J., Isaacson P. G. An immunocytochemical study of T-cell lymphomas using monoclonal and polyclonal antibodies effective in routinely fixed wax embedded tissues. Histopathology. 1986 Dec;10(12):1243–1260. doi: 10.1111/j.1365-2559.1986.tb02568.x. [DOI] [PubMed] [Google Scholar]
- Norton A. J., Isaacson P. G. Detailed phenotypic analysis of B-cell lymphoma using a panel of antibodies reactive in routinely fixed wax-embedded tissue. Am J Pathol. 1987 Aug;128(2):225–240. [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Weiss L. M., Medeiros L. J., Wood G. S., Warnke R. A. Immunophenotypic criteria for the diagnosis of non-Hodgkin's lymphoma. Am J Pathol. 1987 Jul;128(1):181–201. [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Hollema H., Visser L., Vos H. Monoclonal antibodies (MT1, MT2, MB1, MB2, MB3) reactive with leukocyte subsets in paraffin-embedded tissue sections. Am J Pathol. 1987 Jun;127(3):418–429. [PMC free article] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D., Rosen F. S. Biosynthesis of human sialophorins and analysis of the polypeptide core. Biochemistry. 1987 Jun 30;26(13):3908–3913. doi: 10.1021/bi00387a025. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Zimmerman C., Kenney D., Rosen F. S. Expression on blood cells of sialophorin, the surface glycoprotein that is defective in Wiskott-Aldrich syndrome. Blood. 1987 Jul;70(1):104–109. [PubMed] [Google Scholar]
- Said J. W., Stoll P. N., Shintaku P., Bindl J. M., Butmarc J. R., Pinkus G. S. Leu-22: a preferential marker for T-lymphocytes in paraffin sections. Staining profile in T- and B-cell lymphomas, Hodgkin's disease, other lymphoproliferative disorders, myeloproliferative diseases, and various neoplastic processes. Am J Clin Pathol. 1989 May;91(5):542–549. doi: 10.1093/ajcp/91.5.542. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Strickler J. G., Weiss L. M., Copenhaver C. M., Bindl J., McDaid R., Buck D., Warnke R. Monoclonal antibodies reactive in routinely processed tissue sections of malignant lymphoma, with emphasis on T-cell lymphomas. Hum Pathol. 1987 Aug;18(8):808–814. doi: 10.1016/s0046-8177(87)80055-2. [DOI] [PubMed] [Google Scholar]
- Stross W. P., Jones M., Mason D. Y. Automation of APAAP immunocytochemical technique. J Clin Pathol. 1989 Jan;42(1):106–112. doi: 10.1136/jcp.42.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West K. P., Warford A., Fray L., Allen M., Campbell A. C., Lauder I. The demonstration of B-cell, T-cell and myeloid antigens in paraffin sections. J Pathol. 1986 Oct;150(2):89–101. doi: 10.1002/path.1711500203. [DOI] [PubMed] [Google Scholar]