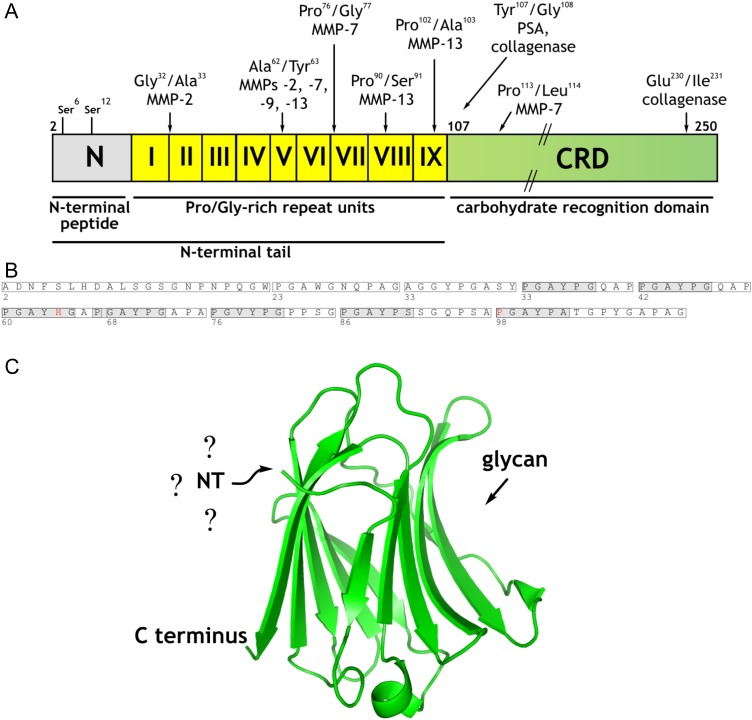
Fig. 1.
(A) Schematic overview of the trimodular design of human Gal-3. The NT is composed of the peptide with two sites for Ser phosphorylation (N) and the nine non-triple helical collagen-like repeat units (I–IX), these being followed by the CRD. Cleavage sites for MMPs, PSA and bacterial collagenase are indicated by arrows (adapted from Kopitz et al. 2014). (B) Amino acid sequence of the NT. Repeats I–IX and 7-fold occurrence of the pentapeptide PGAYP are indicated by boxes and shaded boxes, respectively. (C) Crystal structure of the CRD (PDB code 3ZSJ; Seetharaman et al. 1998). The question marks highlight the current uncertainty about the conformation of the NT, and the arrow identifies the contact site for glycan binding. This figure is available in black and white in print and in color at Glycobiology online.