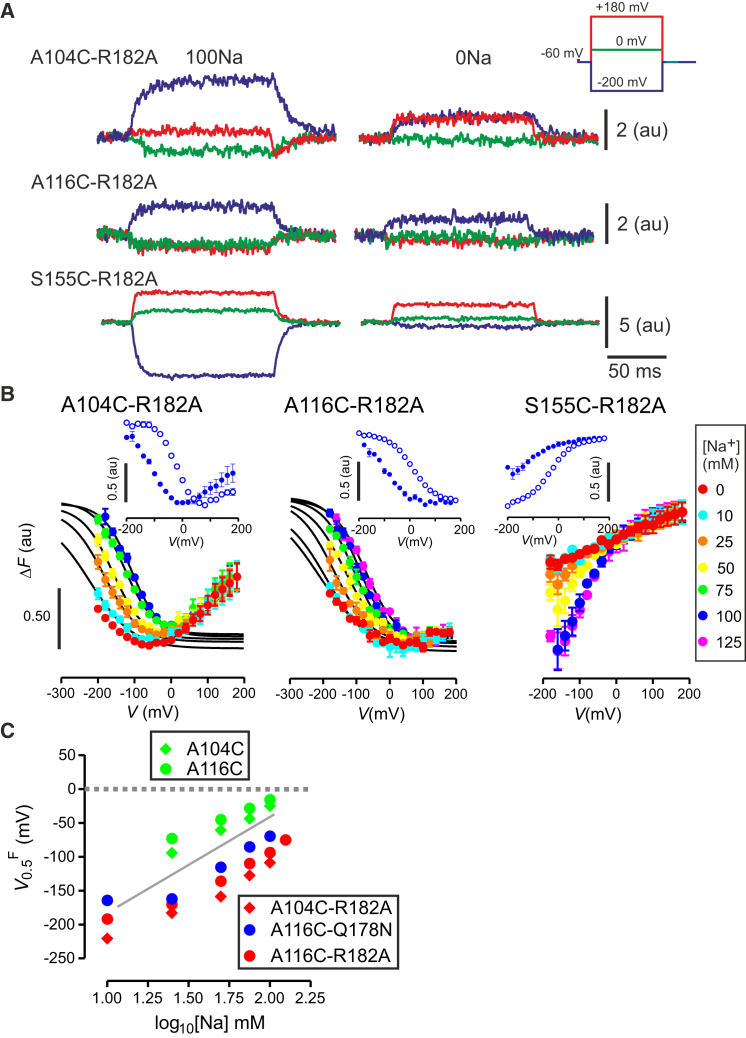
Figure 5.
Voltage-clamp fluorometry dependence of ΔF-V on external Na+ concentration. (A) Representative recordings of changes in fluorescence (ΔF) in response to voltage steps from the –60 mV holding potential to –200, 0, and +180 mV for oocytes containing the Na1 mutation R182A and a substituted Cys at each of the three reporter positions. Oocytes expressing the respective double mutants were labeled with the fluorophore MTS-TAMRA at Cys-104 (upper); Cys-116 (center), and Cys-155 (lower) and recordings were made in the presence (100Na) and absence (0Na) of external Na+ ions. The differences in polarity and magnitude of the change in fluorescence intensity depend on both labeling position and expression levels for the same voltage step. (B) ΔF–V data when superfusing with different [Na+] indicated for the three double mutants. All data sets were superimposed at the depolarizing limit. Continuous lines are fits using Eq. 1 to data sets normalized to 100Na data set in each case. Fitting was only applied to data points lying in the monotonic range of ΔF for each [Na+]. Fitting was not performed for the S155C-R182A data because of the lack of saturation at hyperpolarizing potentials. Each data point represents mean ± SE for n > 5 cells. (Insets) ΔF-V data obtained from a representative oocyte expressing the respective cysteine-only mutant when superfusing with 100Na (open circles) and 0Na (solid circles). (C) Dependence of V0.5Q on [Na+] for the single mutants A104C, A116C (data from Patti and Forster (3)) and double mutants A104C-Q178N, A116C-Q178N, and A116C-R182A obtained in this study. (Shaded line) Theoretical limiting slope of ∼120 mV/decade predicted for 2 Na+ ions binding to protein. Each data point represents mean ± SE for n > 5 cells. To see this figure in color, go online.