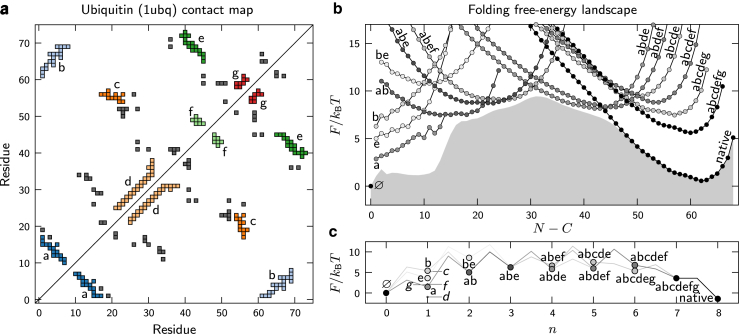
Figure 3.
Predicted folding free-energy landscapes for ubiquitin. (a) The contact map obtained from the crystal structure of ubiquitin (PDB: 1UBQ) indicating the discrete substructures a–g described in the Materials and Methods. (b) The free energy of each topological configuration as a function of the total number of interacting residues, N. The number of structured regions, C, is 1 for all configurations except the unfolded state, Ø, where C = 0. The shaded region shows the one-dimensional free-energy profile. (c) The free energy of each topological configuration as a function of the number of assembled substructures, n. All free energies are calculated relative to the state Ø, and the inverse temperature is tuned to achieve equal stabilities of the native and unfolded ensembles. The shading indicates the fraction of the net folding flux through each configuration. Only configurations with at least 10% of the net folding flux are shown, except in (c), n = 1, where all substructures are labeled. To see this figure in color, go online.