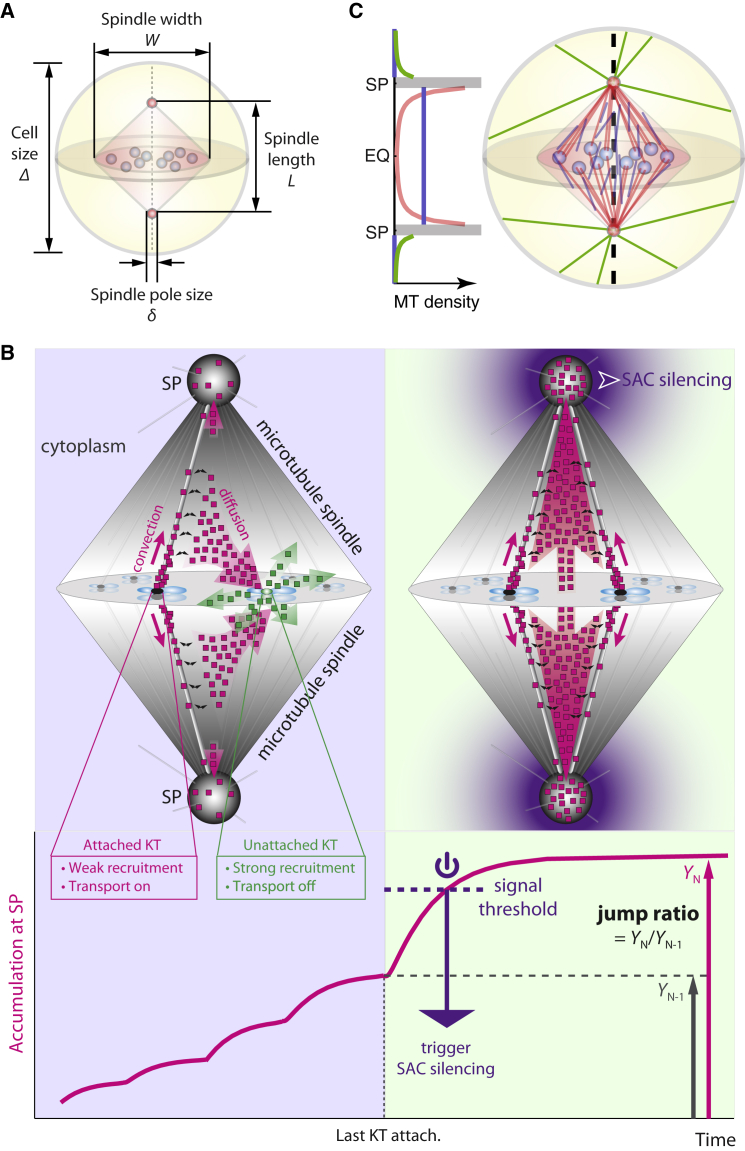
Figure 1.
Summary of model. (A) Notations for relevant geometric dimensions of the cell and the mitotic spindle. (B) Illustrative summary of the spatiotemporal model for SAC silencing. (Upper left panel) Poleward stream of SAC components (magenta squares) that emanated from attached kinetochores (black oval) is strongly diverted by unattached kinetochores (green oval). Unattached kinetochores convert proteins from streaming (magenta squares) to diffusive state (green squares). (Upper right panel) Once all kinetochores are attached, poleward stream becomes free of diversion, leading to significant increase of SAC accumulation at the spindle pole. The signal makes a robust trigger for SAC silencing that propagates throughout the cell. For clarity, fluxes of SAC components are only shown for two pairs of kinetochores in the foreground. Distribution of the squares does not reflect spatial distribution of SAC components. (Translucent magenta arrows) Flux of streaming proteins. (Translucent green arrows) Flux of diffusive proteins. (Solid magenta arrows) Proteins streaming poleward along microtubules. (Black curly arrows) Streaming proteins binding/unbinding with microtubules. (Lower panel) Spindle pole signal (accumulation of SAC components) with successive kinetochore-spindle attachments (magenta solid line). The last kinetochore attachment elevates the signal above the threshold and triggers SAC silencing. The jump ratio is defined as the ratio between the steady-state spindle pole signals after and before the last kinetochore attachment. (C) Illustration of microtubule distribution depicted by the current, improved model. (Red) Spindle microtubules in Group 1 characterized by N1. (Blue) Spindle microtubules in Group 2 characterized by ρ2. (Green) Astral microtubules outside spindle.