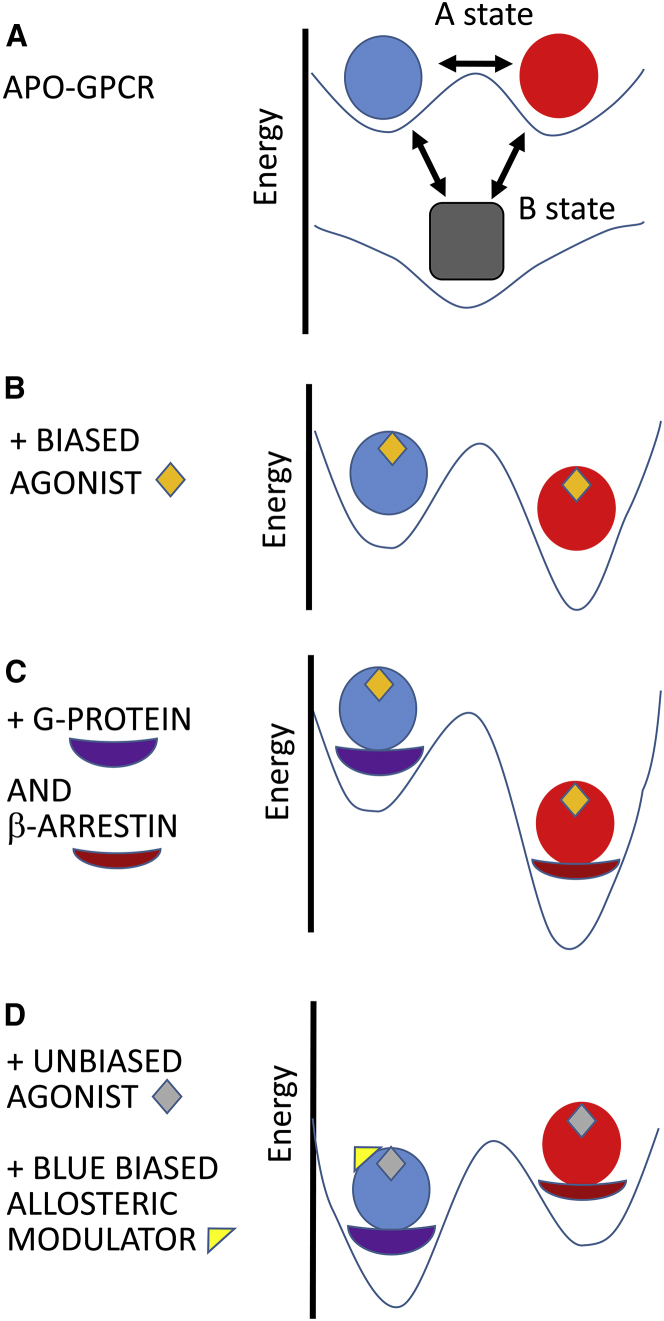
Figure 2.
Energy relationships among the conformational states. (A) Intrinsic equilibrium among the conformations of the GPCR molecules in the absence of agonists, antagonists, transfer proteins, or allosteric modulators. Under these conditions the B state is more stable than the A states (shown here with equal stabilities). (B) The redistribution of Ablue and Ared states in the presence of a biased agonist (in this case favoring the Ared state). Both states are stabilized, but Ared is moreso than Ablue. The B state is not shown. (C) Molecules are shown as in (B), with the addition of transfer proteins, G-protein and β-arrestin. Each transfer protein can bind to both the Ablue and Ared states, but only the preferential binding partners are presented here. An additional stabilization of the Ared state arises from the binding energy provided by β-arrestin. (D) The case of an unbiased agonist (gray diamond) bound in the presence of a biased positive allosteric modulator that favors the Ablue state. To see this figure in color, go online.