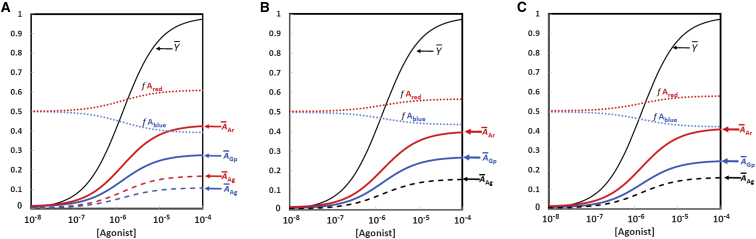
Figure 3.
Simulated dose-response curves to represent clenbuterol. Each graph shows the fractional separation of the A state into red and blue components, given by f Ared and f Ablue, respectively, along with the binding function, . (A) Ratio of β-arrestin/G-protein = 1 (concentration of 5.0 × 10−6 M for both), but with stronger binding of the agonist to the Ared state corresponding to the parameter values in Table 1. The curves are separated for A-state molecules with agonist and G-protein (Ablue), agonist and β-arrestin (Ared), or agonist alone (AAg) shown separately for the Ablue and Ared states. Not shown are the fraction of A states that are fully nonliganded (negligible, ∼10−4) and the B state in its various liganded forms (∼2% at [agonist] = 10−4 M). (B) Ratio of β-arrestin/G-protein = 1, with stronger binding for β-arrestin to the Ared state than for G-protein to the Ablue state. Parameter values are [G-protein] = [β-arrestin] = 2.0 × 10−5 M; other values are as in Table 1, except for AKGp_blue = 3.0 × 10−6 M and AKAr_red = 2.0 × 10−6 M, agonist affinity for both Ared and Ablue = 4.0 × 10−8. For these conditions agonist alone (AAg) is shown in black since the curves for the Ablue and Ared states overlap. (C) Ratio of β-arrestin/G-protein > 1: [G-protein] = 3.0 × 10−6 M, and [β-arrestin] = 5.0 × 10−6 M, with AKGp_blue = AKAr_red = 2.0 × 10−6 M. Other details are as in (B). To see this figure in color, go online.