Abstract
New Candida species may cause bloodstream infections challenging current therapeutic approaches because of unpredictable susceptibility and virulence. In the present report, we describe a fungemia case due to Candida pulcherrima in a premature neonate. After full in vitro diagnostic workup, the neonate was successfully treated with liposomal amphotericin B and micafungin achieving rapid fungal eradication from blood.
Keywords: Candidemia, Neonate, Combination antifungal therapy, Micafungin, Liposomal amphotericin B
1. Introduction
Candida species are the third most frequently isolated pathogens from blood cultures in neonatal late-onset sepsis (9–13%) [1]. The mortality rate due to Candida sepsis is high ranging from 25% to 54% and it can reach 70% in very low birth weight newborns [1], [2]. Candida albicans has been historically the most frequent pathogen in neonates followed by Candida parapsilosis and other Candida species such as Candida tropicalis, Candida glabrata and Candida krusei [3], [4]. However, rare Candida species have been increasingly recognized as potential pathogens for neonates [5]. Admission into a Neonatal Intensive Care Unit quadruples the risk of infection by these pathogens [6]. Given the recognition of increased number of bloodstream infections by uncommon opportunistic yeasts with variable susceptibility to antifungal drugs [7], identification of new potential pathogens is important for initiation of prompt and targeted antifungal therapy.
The most recent guidelines by ESCMID favor the use of amphotericin B (conventional and liposomal), fluconazole and micafungin (B-II) for the treatment of neonatal candidemia [8]. However, antifungal resistance to fluconazole is increased among Candida non-albicans species and particularly C. glabrata, whereas echinocandin resistance among C. glabrata isolates poses a therapeutic challenge in the treatment of candidemia [9]. While C. albicans and C. parapsilosis constitute the great majority of Candida species causing neonatal candidiasis, rare yeasts with variable susceptibility can occasionally be found and require special care [10].
In the present case report, we describe a rare case of fungemia by Candida pulcherrima in a premature neonate together with the diagnostic and therapeutic approaches followed.
2. Case
A male newborn born as a gemini B twin with a gestation age of 33 weeks was admitted to the Neonatal Intensive Care Unit at the General Hospital of Nikaia, Athens, Greece due to prematurity and respiratory distress syndrome. The neonate was delivered via spontaneous vaginal delivery following premature rupture of the amniotic membrane. The birth weight was 2080 g. He was initially treated empirically with ampicillin and gentamicin. All drugs were administered via a peripheral catheter, which was changed every three days. Parenteral nutrition was administered until day 3.
On day 0 he developed symptoms and sign of sepsis with fever to 38°C, paleness, indolence and acrocyanosis. His laboratory results demonstrated thrombocytopenia (min 21,000/mm3) and increased CRP (max 51 mg/L). The antibiotic therapy was modified to meropenem and teicoplanin and on day 3 liposomal amphotericin B (7 mg/kg/d. i.v.) was added and maintained throughout the treatment after fungal growth was detected in four aerobic blood bottles (BacT Alert, Biomerieux, France) collected on day 0. C. pulcherrima was identified as described below and it was detected in all blood cultures collected on days 3, 6 and 7. Ultrasound of the head and abdomen, lumbar puncture, urine culture, ophthalmologic exam and echocardiogram did not indicate disseminated candidiasis.
Four days after initiation of liposomal amphotericin B, the blood cultures remained positive for the same yeast and micafungin 10 mg/kg/d i.v. was added on day 7. His general condition was improved progressively, CRP levels decreased (<3 mg/L) and after two days of combined antifungal therapy on day 9 the blood cultures became negative. The treatment continued for another 16 days. On day 30, the neonate was discharged from the hospital in good condition and with normal laboratory results.
2.1. Species identification
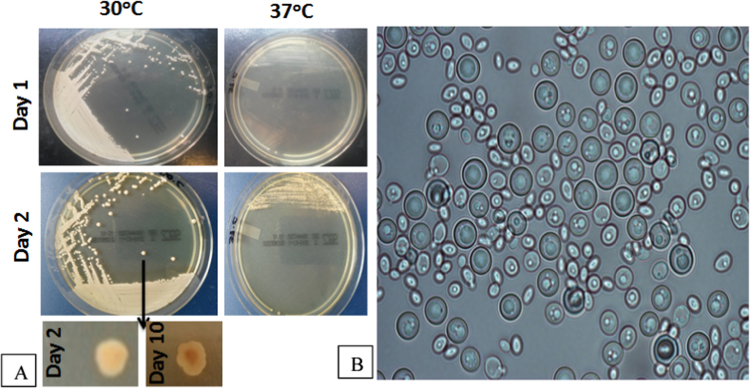
The isolate grew on Sabouraud Dextrose agar plates slowly at 37 °C and best at 25–30 °C (Fig. 1A). The colonies were slow growing, convex, cream colored with a reddish pigment developed after 48 h (Fig. 1A). Microscopically ovoid to ellipsoidal budding yeasts with chlamydospores but no pheudohyphae were found (Fig. 1B). Biochemical identification with VITEK 2 Compact automated system (Biomerieux, France) revealed C. pulcherrima (good identification with 90% confidence level). Identification was confirmed with ITS sequencing as previously described using ITS1 (5- TCCGTAGGTGAACCTGCGG-3), and ITS4 (5-TCCTCCGCTTATTGATATGC-3) primers (Fig. 2) [11]. High sequence alignment (99%) was found in Genbank Blast analysis with Metschnikowia pulcherrima, the sexual name of C. pulcherrima (GenBank Accession No KX276090).
Fig. 1.
Macroscopic (A) and microscopic (B) photos of C. pulcherrima. A. Cream colored colonies with reddish pigment on the reverse of Sabouraud Dextrose Agar. B. Chlamydospores, budding yeast and no pseudohyphae were observed in corn-meal agar after incubation at 37 °C for 48 h. (Magnification 400×).
Fig. 2.
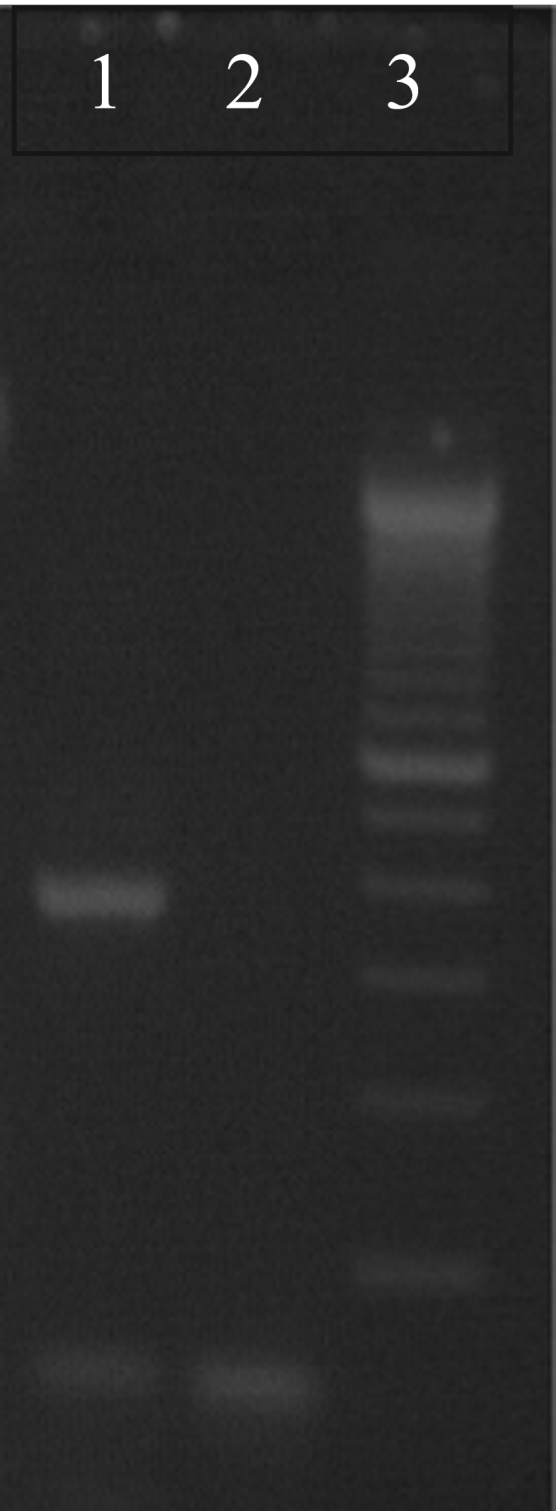
Gel electrophoresis of PCR products after amplification of C. pulcherrima DNA with ITS1 (5-TCCGTAGGTGAACCTGCGG-3) and ITS4 (5-TCCTCCGCTTATTGATATGC-3) primers, 1: C. pulcherrima 368 bp PCR product, 2: negative control, 3: 100 bp DNA ladder.
2.2. In vitro susceptibility testing
In vitro antifungal susceptibility was tested with Sensititre YeastOne and the minimal inhibitory concentrations (MICs) were for amphotericin B 0.5 mg/L, for fluconazole 0.25 mg/L, for itraconazole 0.03 mg/L, for voriconazole ≤0.008 mg/L, for posaconazole ≤0.008 mg/L, for flucytosine ≤0.06 mg/L, for micafungin 0.12 mg/L, for anidulafungin 0.25 mg/L and for caspofungin 0.5 mg/L. In vitro susceptibility to amphotericin B and micafungin were verified with the EUCAST method [12] with 24/48 h MIC of <=0.03/0.125 mg/L and 0.015/0.06 mg/L at 37 °C, and 0.06/0.125 mg/L and 0.06/0.25 mg/L at 30 °C, respectively. The MIC of liposomal amphotericin B was one two-fold dilution lower. The minimal fungicidal concentration was determined as the lowest concentration with no viable cells after subculturing 100 μL from the clear (no visible growth) wells after 48 h at 30 °C and they were 0.5 mg/L for amphotericin B and >8 mg/L for micafungin. No killing was observed at concentrations ≤0.125 mg/L.
2.3. In vitro combination testing
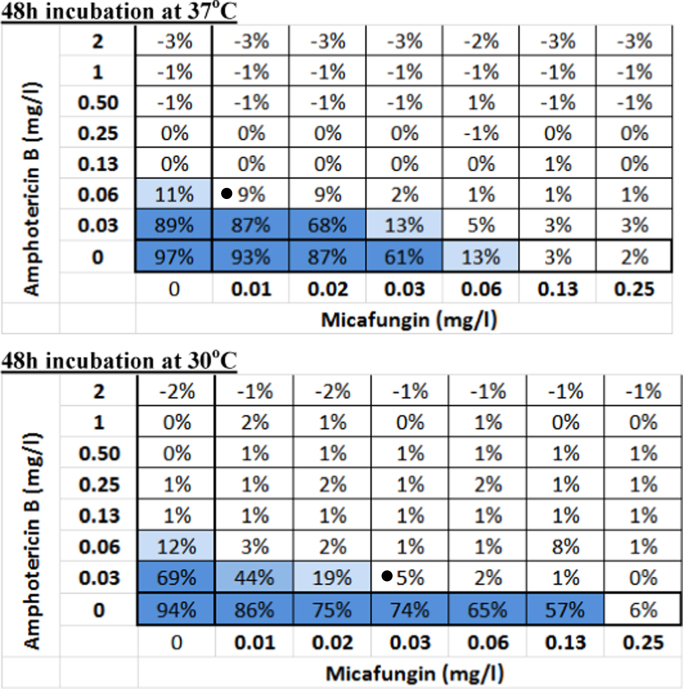
In vitro interaction between amphotericin B and micafungin was determined with a checkerboard broth microdilution as previously described [13]. The combination using 10% growth inhibition endpoint after 48 h at 37 °C and 30 °C was additive and synergistic with a Fractional Inhibitory Index of 0.56 and 0.375, respectively, reducing the MICs of both drugs from 0.125 to 0.06 mg/L for amphotericin B and to 0.01 mg/L for micafungin at 37 °C. A similar decrease was found at 30 °C (Fig. 3). When clear wells were subcultured in order to determine the fungicidal activities the cfu/mL at 0.25 mg/L of amphotericin B were decreased from 2×103 to 0.5×103 cfu/ml when combined with 0.03–0.25 mg/L of micafungin at 30 °C.
Fig. 3.
In vitro checkerboard of amphotericin B + micafungin after 48 h incubation at 37 °C (top panel) and 30 °C (bottom panel). The Fractional Inhibitory Index using the <10% growth endpoint was 0.56 and 0.375, respectively (black dots).
3. Discussion
C. pulcherrima is an environmental, saprophytic yeast but also an opportunistic pathogen. It was isolated from skin lesions and nails [14], [15]. It is member of the Metschnikowiaceae family and its morphology and physiology are very close to those of C. lusitaniae [14]. This is the second case of neonatal fungemia due to this yeast [16] whereas recently a case of community acquired fungemia caused by C. pulcherrima in an injection-drug user was reported [17]. A C. pulcherrima blood-stream infection in healthcare setting was related to the use of indwelling catheter for parenteral nutrition [18].
Premature neonates are at particularly increased risk to develop invasive candidiasis with excessive case fatality due to their low birth weight, poor nutrition, enteral malabsorption, insufficient microbial defenses and underdeveloped anatomic barriers. In premature neonates as many as 80% of cases have occurred during the first 42 days of life. Birth weight and postnatal age at the time of infection also predict subsequent mortality. Major risk factors for fungemia include intravascular catheters, parenteral hyperalimentation and broad spectrum antibiotics [1], [4], [6]. In our case prematurity (gestation age of 33 weeks), low birth weight (<2500 g), insufficient immune system and underdeveloped anatomic barriers were the predisposing factors for candidemia. Horizontal transmission via contaminated medical devices, fluids or the hands of health care workers may be the sources [19].
The first case of C. pulcherrima fungemia occurred in a neonate by an isolate with fluconazole MIC of 2 mg/L and amphotericin B MIC 0.004 mg/L. It was initially treated with fluconazole (6 mg/kg/d) but because of positive blood cultures, amphotericin B lipid complex (5 mg/kg/d) was initiated. After 6 days of treatment the general condition was improved but only after 15 days of treatment the blood cultures became negative [16]. The second case of C. pulcherrima fungemia occurred in an adult by an isolate with caspofungin MIC 0.25 mg/L. It was treated with caspofungin (70 mg/d) and yeast was eradicated at the second day of treatment. Both cases are in line with the present case where amphotericin B failed to eradicate the yeast after 5 days of treatment whereas rapid eradication was observed when micafungin was added. In vivo enhancement of liposomal amphotericin B efficacy when combined with micafungin was previously reported for the treatment of azole-refractory C. guillieremondii fungemia which failed liposomal amphotericin B plus voriconazole combination therapy [20].
A synergistic effect between amphotericin B and micafungin could explain the rapid eradication when micafungin was added to liposomal amphotericin B therapy. The two-fold reduction of amphotericin B MIC when combined with micafungin could enhance the efficacy of liposomal amphotericin B in order to reach the PKPD target tCmax/MIC 70 associated with complete response in children. This MIC reduction is particularly important when serum levels are at the lower end of 11–44 mg/L achieved in children with candidemia although neonates may have different pharmacokinetics [21]. In addition, since liposomal amphotericin B is >99% protein bound, serum free concentrations may be lower than the in vitro MFC determined in the present study (0.5 mg/L) but sufficient to kill the yeast when combined with micafungin (0.25 mg/L). Persistent Candida fermentati fungemia during liposomal amphotericin B therapy was successfully treated previously with the combination of liposomal amphotericin B with caspofungin in a preterm neonate [22]. However, given that the mean duration time of fungal eradication after liposomal amphotericin B therapy of neonatal candidiasis is 9 days [23], the eradication observed in the present study after micafungin was added may be a coincidence with late fungicidal activity of liposomal amphotericin B. Another explanation of rapid eradication could be due to micafungin monotherapy, of which efficacy is shown in clinical trials [24]. Despite the higher MFC, its in vivo effect may be enhanced by the strong immunomodulatory effects that echinocandins possess decreasing dysregulated cytokines/chemokines [25]. Finally, since Candida species easily form biofilms in catheters, the combination effect may be due to local control of biofilm although in our case no central catheters were present. Although liposomal amphotericin B possesses antibiofilm activity against C. lusitaniae at lower concentrations than micafungin (sMIC 0.125 vs >2048 mg/L), micafungin was found to damage more mature biofilms than liposomal amphotericin B at high concentrations achieved locally during infusion [26]. Thus, the combination of liposomal amphotericin B+micafungin may be used for rapid eradication of C. pulcherrima in bloodstream infections.
Conflict of interest
None.
Acknowledgments
None.
References
- 1.Benjamin D.K., Stoll B.J., Fanaroff A.A., McDonald S.A., Oh W., Higgins R.D., Duara S., Poole K., Laptook A., Goldberg R. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 2.das L., Miranda N., Rodrigues E.C.A., Costa S.F., van der Heijden I.M., Dantas K.C., Lobo R.D., Basso M., Varkulja G.F., Krebs V.L.J., Gibelli M.A.B.C., Criado P.R., Levin A.S. Candida parapsilosis candidaemia in a neonatal unit over 7 years: a case series study. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran C., Grussemeyer C.A., Spalding J.R., Benjamin D.K., Reed S.D. Candida albicans and non-albicans bloodstream infections in adult and pediatric patients: comparison of mortality and costs. Pediatr. Infect. Dis. J. 2009;28:433–435. doi: 10.1097/INF.0b013e3181920ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juyal D., Sharma M., Pal S., Rathaur V.K., Sharma N. Emergence of non-albicans Candida species in neonatal candidemia. N. Am. J. Med. Sci. 2013;5:541–545. doi: 10.4103/1947-2714.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinbach W.J., Roilides E., Berman D., Hoffman J.A., Groll A.H., Bin-Hussain I., Palazzi D.L., Castagnola E., Halasa N., Velegraki A., Dvorak C.C., Charkabarti A., Sung L., Danziger-Isakov L., Lachenauer C., Arrieta A., Knapp K., Abzug M.J., Ziebold C., Lehrnbecher T., Klingspor L., Warris A., Leckerman K., Martling T., Walsh T.J., Benjamin D.K., Zaoutis T.E. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr. Infect. Dis. J. 2012;31:1252–1257. doi: 10.1097/INF.0b013e3182737427. [DOI] [PubMed] [Google Scholar]
- 6.Trofa D., Gácser A., Nosanchuk J.D. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 2008;21:606–625. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taj-Aldeen S.J., AbdulWahab A., Kolecka A., Deshmukh A., Meis J.F., Boekhout T. Uncommon opportunistic yeast bloodstream infections from Qatar. Med. Mycol. 2014;52:552–556. doi: 10.1093/mmycol/myu016. [DOI] [PubMed] [Google Scholar]
- 8.Hope W.W., Castagnola E., Groll A.H., Roilides E., Akova M., Arendrup M.C., Arikan-Akdagli S., Bassetti M., Bille J., Cornely O.A., Cuenca-Estrella M., Donnelly J.P., Garbino J., Herbrecht R., Jensen H.E., Kullberg B.J., Lass-Flörl C., Lortholary O., Meersseman W., Petrikkos G., Richardson M.D., Verweij P.E., Viscoli C., Ullmann A.J. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin. Microbiol. Infect. 2012;18(Suppl 7):S38–S52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 9.Cleveland A.A., Harrison L.H., Farley M.M., Hollick R., Stein B., Chiller T.M., Lockhart S.R., Park B.J. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008–2013: results from population-based surveillance. PLoS One. 2015;10:e0120452. doi: 10.1371/journal.pone.0120452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Matta D.A., de Almeida L.P., Machado A.M., Azevedo A.C., Kusano E.J.U., Travassos N.F., Salomão R., Colombo A.L. Antifungal susceptibility of 1000 Candida bloodstream isolates to 5 antifungal drugs: results of a multicenter study conducted in São Paulo, Brazil, 1995–2003. Diagn. Microbiol. Infect. Dis. 2007;57:399–404. doi: 10.1016/j.diagmicrobio.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 11.White T., Bruns T., Lee S., Taylor J. Analysis of phylogenetic Relationships by amplification and Direct sequencing of ribosomal RNA Genes. In: Innis MA S.J. and W.T., Gelfand D.H., editors. PCR Protoc. a Guid. to Methods Appl. Academic Press; New York, NY: 1990. pp. 315–322. [Google Scholar]
- 12.EUCAST EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Micro. Infect. 2008;14:398–405. doi: 10.1111/j.1469-0691.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 13.Meletiadis J., Pournaras S., Roilides E., Walsh T.J. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2010;54:602–609. doi: 10.1128/AAC.00999-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pospisil L. The significance of Candida pulcherrima findings in human clinical specimens. Mycoses. 1989;32:581–583. 〈http://www.ncbi.nlm.nih.gov/pubmed/2615783〉 (accessed 15.05.16) [PubMed] [Google Scholar]
- 15.Noël T., Favel A., Michel-Nguyen A., Goumar A., Fallague K., Chastin C., Leclerc F., Villard J. Differentiation between atypical isolates of Candida lusitaniae and Candida pulcherrima by determination of mating type. J. Clin. Microbiol. 2005;43:1430–1432. doi: 10.1128/JCM.43.3.1430-1432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bereczki L., Bartha N., Kocsubé S., Sóki J., Lengyel G., Tálosi G., Máder K., Deák J., Dóczi I. Fungaemia caused by Candida pulcherrima. Med. Mycol. 2012;50:522–524. doi: 10.3109/13693786.2011.644590. [DOI] [PubMed] [Google Scholar]
- 17.Deconinck L., Meybeck A., Pradier M., Patoz P., Melliez H., Senneville E. Community acquired fungemia caused by Candida pulcherrima: diagnostic contribution of MALDI-TOF mass spectrometry. Ann. Clin. Microbiol. Antimicrob. 2016;15:14. doi: 10.1186/s12941-016-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A. Weber, S. Kolb, The repeated isolation of Candida pulcherrima (Lindner) Windisch from blood cultures of a patient on parenteral nutrition, Mykosen. 29, 1986, pp. 127–131. 〈http://www.ncbi.nlm.nih.gov/pubmed/3084972〉 (accessed 15.05.16). [PubMed]
- 19.Juyal D., Adekhandi S., Negi V., Sharma N. An Outbreak of Neonatal Candidemia Due to Non-albicans Candida Species in a Resource Constrained Setting of Uttarakhand State, India. J. Clin. Neonatol. 2013;2:183–186. doi: 10.4103/2249-4847.123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.T. Saitoh, T. Matsushima, H. Shimizu, Y. Osaki, A. Yamane, H. Irisawa, A. Yokohama, H. Uchiumi, H. Handa, N. Tsukamoto, M. Karasawa, Y. Nojima, H. Murakami, Successful treatment of azole-refractory Candida guilliermondii fungemia with a combination therapy of micafungin and liposomal amphotericin B., (Rinshō Ketsueki), Japanese J. Clin. Hematol. 49, 2008, pp. 94–98. 〈http://www.ncbi.nlm.nih.gov/pubmed/18341039〉 (accessed 15.05.16). [PubMed]
- 21.Hong Y., Shaw P.J., Nath C.E., Yadav S.P., Stephen K.R., Earl J.W., McLachlan A.J. Population pharmacokinetics of liposomal amphotericin B in pediatric patients with malignant diseases. Antimicrob. Agents Chemother. 2006;50:935–942. doi: 10.1128/AAC.50.3.935-942.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Sweih N., Ahmad S., Joseph L., Khan S., Vayalil S., Chandy R., Khan Z. Candida fermentati as a cause of persistent fungemia in a preterm neonate successfully treated by combination therapy with amphotericin B and caspofungin. J. Clin. Microbiol. 2015;53:1038–1041. doi: 10.1128/JCM.03351-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juster-Reicher A., Leibovitz E., Linder N., Amitay M., Flidel-Rimon O., Even-Tov S., Mogilner B., Barzilai A. Liposomal amphotericin B (AmBisome) in the treatment of neonatal candidiasis in very low birth weight infants. Infection. 2016;28:223–226. doi: 10.1007/s150100070040. 〈http://www.ncbi.nlm.nih.gov/pubmed/10961528〉 (accessed 21.05.16) [DOI] [PubMed] [Google Scholar]
- 24.Manzoni P., Wu C., Tweddle L., Roilides E. Micafungin in premature and non-premature infants: a systematic review of 9 clinical trials. Pediatr. Infect. Dis. J. 2014;33:e291–e298. doi: 10.1097/INF.0000000000000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baltch A.L., Lawrence D.A., Ritz W.J., Andersen N.J., Bopp L.H., Michelsen P.B., Carlyn C.J., Smith R.P. Effects of echinocandins on cytokine/chemokine production by human monocytes activated by infection with Candida glabrata or by lipopolysaccharide. Diagn. Microbiol. Infect. Dis. 2012;72:226–233. doi: 10.1016/j.diagmicrobio.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Simitsopoulou M., Kyrpitzi D., Velegraki A., Walsh T.J., Roilides E. Caspofungin at catheter lock concentrations eradicates mature biofilms of Candida lusitaniae and Candida guilliermondii. Antimicrob. Agents Chemother. 2014;58:4953–4956. doi: 10.1128/AAC.03117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]