Abstract
The social patterning of cytomegalovirus (CMV) and its implication in aging suggest that the virus may partially contribute to socioeconomic disparities in mortality. We used Cox regression and inverse odds ratio weighting to quantify the proportion of the association between socioeconomic status (SES) and all-cause mortality that was attributable to mediation by CMV seropositivity. Data were from the National Health and Nutrition Examination Survey (NHANES) III (1988–1994), with mortality follow-up through December 2011. SES was assessed as household income (income-to-poverty ratio ≤1.30; >1.30 to ≤1.85; >1.85 to ≤3.50; >3.50) and education (<high school; high school; >high school). We found strong associations between low SES and increased mortality: hazard ratio (HR) 1.80; 95% confidence interval (CI): 1.57, 2.06 comparing the lowest versus highest income groups and HR 1.29; 95% CI: 1.13, 1.48 comparing <high school versus >high school education. 65% of individuals were CMV seropositive, accounting for 6–15% of the SES-mortality associations. Age modified the associations between SES, CMV, and mortality, with CMV more strongly associated with mortality in older individuals. Our findings suggest that cytomegalovirus may partially contribute to persistent socioeconomic disparities in mortality, particularly among older individuals.
Keywords: Aging, Cytomegalovirus, Mortality, NHANES III, Socioeconomic status
1. Introduction
In 2013, over 45 million Americans were living in poverty (DeNavas-Walt and Proctor, 2014) and 24.5 million of those over the age 25 had not completed high school (Census Bureau, 2013). Due to a complex and interrelated set of social, behavioral, and biological processes—both historical and contemporary—individuals with a lower socioeconomic status (SES) persistently suffer a disproportionate burden of premature mortality associated with a range of health conditions and have a lower overall life expectancy (Deaton, 2016; Chetty et al., 2016; Adler and Newman, 2002; Braveman et al., 2010; Meyer et al., 2013; Hummer and Hernandez, 2013; National Center for Health Statistics Health, 2011; Geronimus et al., 2011; Krueger et al., 2015; Muennig et al., 2010; Olshansky et al., 2012). While SES is clearly linked to mortality, the mechanisms underlying this disparity remain poorly understood.
One potential, yet under investigated, pathway through which socioeconomic disadvantage may “get under the skin” to impact mortality is through differential pathogen exposure across the life course. Prior studies have shown that low SES is associated with both seropositivity for (Bate et al., 2010; Cannon et al., 2010; Colugnati et al., 2007; Dowd et al., 2009a; Dowd et al., 2009b; Staras et al., 2006; Simanek et al., 2009) and immune control of (Dowd et al., 2008; Dowd and Aiello, 2009; Dowd et al., 2012) the herpesvirus cytomegalovirus (CMV), a pathogen that once acquired persists in a latent state but is capable of reactivation. A parallel body of evidence suggests that seropositivity to and reactivation of CMV may play a key role in long-term health outcomes. Indeed, the virus has been implicated in the etiology of numerous chronic disease outcomes including cardiovascular disease, cognitive and physical decline, depression and cancer (Simanek et al., 2009; Aiello et al., 2006; Harkins et al., 2002; Itzhaki et al., 2004; Liu et al., 2006; Samanta et al., 2003; Schmaltz et al., 2005; Sorlie et al., 2000; Simanek et al., 2014; Tarter et al., 2014). Moreover, in previous population-based studies by the authors as well as others, CMV seropositivity has been shown to predict all-cause mortality (Gkrania-Klotsas et al., 2013; Roberts et al., 2010; Simanek et al., 2011). The exact biological mechanisms by which CMV may impact health are still under investigation, but a growing body of evidence suggests that subclinical reactivation of the virus over the life course triggers clonal expansion of CMV-specific memory T-cells, ultimately contributing to overall age-related declines in immune function and increased levels of inflammation (Derhovanessian et al., 2011; Hadrup et al., 2006; Khan et al., 2002; Pawelec, 2013; Pawelec et al., 2009). The impact of CMV-driven immunosenescence may already be apparent in younger people and potentially accelerate as the individual ages (Turner et al., 2014). This is consistent with recent findings that CMV infection may enhance responses to influenza vaccination in young people but be detrimental in the elderly (Furman et al., 2015).
The social patterning of CMV and its implication in long-term health outcomes, including mortality, suggest that infection with the pathogen may partially contribute to socioeconomic disparities in mortality. While previous studies have identified CMV seropositivity as a key mediator of the association between SES and specific chronic disease outcomes (Simanek et al., 2009), to our knowledge, no studies have quantified the role of CMV as a mediator between SES and all-cause mortality at the population level. Using data from a nationally representative sample of US adults, we assessed whether two factors that influence socioeconomic status—household income and educational attainment—were associated with all-cause mortality, and moreover quantified the proportions of these associations that were attributable to mediation by CMV seropositivity. In addition, we examined whether the pathways linking socioeconomic status, CMV seropositivity, and mortality were modified by age.
2. Methods
2.1. Study population
The data for the present study were from the National Health and Nutrition Examination Survey (NHANES) III (1988–1994), a population-based survey based on a multistage stratified probability sample. NHANES III was conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) and was designed to provide nationally representative estimates of the civilian noninstitutionalized US population. Full details on the NHANES III study design and response rates have been published previously (Anon, 1994).
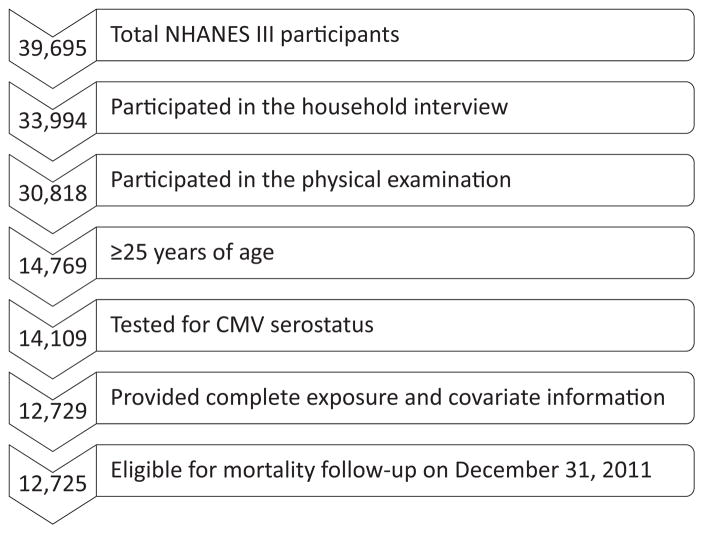
Of the 39,994 individuals who participated in the NHANES III, all individuals who completed both the household interview and the physical examination (n = 30,818) were eligible for inclusion in the present analysis (see Fig. 1). To focus on adult socioeconomic status, we limited our analysis to individuals who were at least 25 years of age (n = 14,769), as has been done in prior studies (Dowd and Aiello, 2009). Fully adjusted models were further limited to those who had been tested for CMV and those who had complete information on household income and study covariates (n = 12,729). Four additional individuals had insufficient identifying data to confirm their mortality status in the National Death Index, resulting in a final sample size of 12,725.
Fig. 1.
Study Flow.
2.2. Measures
2.2.1. Socioeconomic status
The primary exposure in the present study was socioeconomic status assessed as household income and educational attainment. Household income was measured by the income-to-poverty ratio (IPR), which was calculated by dividing total annual household income by the annual poverty threshold as determined by the US Census Bureau based on household size (for example, an IPR of 1.5 indicates that the family income is 1.5 times the poverty threshold). As prior studies strongly suggest that the income-mortality gradient is non-linear (Dowd et al., 2011), we created four categories for household income using the US Department of Agriculture’s food assistance program’s income eligibility cut-points for free (IPR≤1.30) or reduced (IPR ≤1.85) school lunches as recommended in the NHANES III Analytic and Reporting Guidelines (National Center for Health Statistics, 1996): low (IPR ≤1.30), low-middle (IPR >1.30 to ≤1.85), middle (IPR >1.85 to ≤3.50), and high (IPR >3.50). Educational attainment was originally assessed as the number of years of completed education, which we categorized as: less than high school (0 to <12 years), high school (12 years), and more than high school (13+ years) based on recommended cut-points in the NHANES III guidelines (National Center for Health Statistics, 1996).
2.2.2. Mortality status
To ascertain participants’ mortality status, we linked the NHANES III interview, examination, and laboratory data to the Public-use Linked Mortality File, which includes vital statistics for survey participants 18 years of age and older from the date of survey participation through December 31, 2011 (National Center for Health Statistics, 2015). All NHANES III participants with sufficient identifying information to confirm their mortality status in the National Death Index were included in the Public-use Linked Mortality File. Full details on the linkage process have been described previously (National Center for Health Statistics, 2011).
2.2.3. CMV serostatus
CMV serostatus (positive or negative) was the mediating pathway of interest. CMV specific immunoglobulin G (IgG) antibody levels were measured at the CDC in stored sera of NHANES III participants with an Enzyme Linked Immunosorbent Assay (ELISA) (Quest International, Inc., Miami FL). Sera with values near the ELISA cutoff were confirmed with a second ELISA assay (bioMerieux, Inc., Durham, NC). If results of the initial and confirmatory tests disagreed, an Immunofluorescence Assay (IFA) (Bion International, Inc.) was used and the result from this assay was taken as the final test result. This algorithm achieved 98% sensitivity and 99% specificity (Staras et al., 2006).
2.2.4. Covariates
Potential confounders of the association between SES and mortality were assessed via a directed acyclic graph (Greenland et al., 1999) and included the following sociodemographic variables: age (years), gender (female or male), race/ethnicity (non-Hispanic Black, non-Hispanic White, Mexican American, or Other), country of birth (US or other), marital status (married/cohabitating, widowed/separated/divorced, or never married), geographic region (Northeast, Midwest, South, or West), and urbanicity (rural or urban). In age-stratified analyses, age was categorized as 25–39, 40–59, and 60+ years and was otherwise modeled flexibly with restricted quadratic splines using the macro described by Howe et al., (Howe et al., 2011) with knots at the 5th, 35th, 65th, and 95th percentiles and slight modifications to account for the survey design.
Other covariates hypothesized to be mediators of the association between SES and mortality included cigarette smoking, alcohol use, body mass index (BMI), and current chronic health conditions. Cigarette smoking was categorized as current, former, or never. Alcohol use was dichotomized as current or no use. BMI was categorized base on established clinical cut-points: <18.5 (underweight), 18.5 to <25 (normal or healthy weight), 25 to <30 (overweight), or ≥30 (obese) (Schmaltz et al., 2005). Current chronic health conditions were measured with a modified Charlson index that incorporated information on the following comorbidities: myocardial infarction, cerebrovascular/peripheral vascular disease, chronic pulmonary disease, connective tissue/autoimmune disease, ulcer, liver disease, diabetes, moderate to severe renal disease, and any tumor (Dowd and Aiello, 2009; Charlson et al., 1987). Points were assigned for each comorbidity and summed to create a final score as previously described (Charlson et al., 1987), with a higher score indicating a more sever burden of chronic health conditions. Scores were then categorized into three levels: ≤4, >4 to ≤5, and >5. We did not include these potentially mediating health behaviors and biomedical risk factors in the adjustment set of our primary analysis that assessed the association between the SES variables and all-cause mortality due to the risk of introducing bias by conditioning on intermediate variables (e.g., over-adjustment or collider-stratification bias) (Schisterman et al., 2009). However, we did assess these variables in relation to CMV serostatus in a secondary analysis in order to evaluate our assumption of no mediator-outcome confounding, as described below.
2.3. Statistical analysis
All analyses were conducted in SAS 9.4 (SAS Institute, Inc., Cary, North Carolina) and weighted to account for the NHANES III complex survey sampling and non-response (National Center for Health Statistics, 1996). To preserve the complex survey design, the DOMAIN statement was used in SAS survey procedures to obtain results for the specific age group eligible for inclusion in the present study (i.e., 25+ years of age) and for all stratified analyses. To facilitate domain analyses, pseudo data was created for the ineligible subgroup (i.e., <25 years of age), as many of the analysis variables were unavailable for a large portion of this group (e.g., the NHANES III Public-use Linked Mortality File does not include participants <18 years of age) and the data values for observations in one domain have no impact on the results for the other domains. Missing data for the domain of interest (see Fig. 1) were accounted for in the computation of variance estimates by specifying the NOM-CAR (Not Missing Completely at Random) option in SAS (Berglund, 2016). Tests of statistical significance were 2-sided and the threshold for statistical significance was P < 0.05.
Baseline was defined as the NHANES examination date and follow-up continued until the date of death or December 31, 2011, whichever came first. Descriptive statistics were used to characterize the study population at baseline, overall and within strata of SES and CMV serostatus. ANOVA F tests were used to assess differences between strata for continuous variables and Rao-Scott adjusted Pearson chi-square tests (Rao and Scott, 1984) were used for categorical variables. The Breslow estimator (Breslow, 1972) was used to estimate the cumulative probability of all-cause mortality within strata of the SES variables, adjusting for age, gender, race/ethnicity, country of birth, education, marital status, geographic region, and urbanicity. The same approach was used to estimate the cumulative probability of all-cause mortality by CMV serostatus, additionally adjusting for the SES variables.
To quantify the overall association between SES and all-cause mortality, Cox Proportional Hazard models were used to generate covariate-adjusted hazard ratios, as well as corresponding 95% confidence intervals (CI) using the Taylor series linearization method (Binder, 1983) for variance estimation. Both SES variables (household income and educational attainment) were included in the same model. The Efron approximation method was used to handle ties. To assess mediation by CMV seropositivity, we implemented the inverse odds ratio weighting (IORW) technique described by Nguyen et al. (Nguyen et al., 2015) to decompose the overall association between the SES variables and all-cause mortality into the indirect effect mediated through CMV seropositivity and a second component that represented the effect of SES through all other pathways (i.e., the direct effect). This was achieved through a stepwise process. First, logistic regression was used to estimate the odds ratio for the association between the SES variables and CMV seropositivty. The inverse of this association was then used to weight the Cox Proportional Hazards models for the association between the SES variables and mortality, which provided an estimate of the direct effect of the SES variables on mortality not mediated through CMV seropositivity. The indirect effect HR mediated through CMV seropositivity was then obtained by taking the exponentiated difference between the model coefficients for the total and direct effects. If the indirect effects were found to be statistically significant, the proportion of the total effect that was attributable to mediation by CMV seropositivity was calculated by dividing the model coefficients for the indirect effects by the model coefficients for the total effects. Percentile confidence intervals for the indirect effects and proportions mediated were obtained from 1000 bootstrap samples. To determine if the overall, direct, and indirect effects of SES on mortality were modified by age, results were compared across strata of age (25–39, 40–59, and 60+ years).
One of the necessary assumptions for the mediation analysis is no confounding of the mediator-outcome association conditional on covariates (Nguyen et al., 2015). This assumption may be violated if, for example, there are additional factors that affect both CMV serostatus and mortality. To further assess the assumption of no mediator-outcome confounding, in a sensitivity analysis we used logistic regression to estimate the association of four health behavior and biomedical risk factors (cigarette smoking, alcohol use, BMI, and current chronic health conditions) with CMV seropositivity adjusting for covariates included in the primary analyses (age, gender, race/ethnicity, country of birth, education, household income, marital status, geographic region, and urbanicity). A null association between a risk factor and CMV seropositivity was taken as evidence that the risk factor did not strongly confound the CMV-mortality association.
3. Results
3.1. Sample characteristics
Participant characteristics at the time of the NHANES examination are shown in Table 1. Participants were a median of 43 years of age (interquartile range [IQR]: 33–59 years), approximately half (52.5%) were female, and the majority reported their race/ethnicity as non-Hispanic White (77.4%) or non-Hispanic Black (10.6%). Overall, 28.2% had a household income that was low (IPR ≤1.30) or low-middle (IPR >1.30 to ≤1.85) and 58.7% had achieved at most a high school education. Age, gender, race/ethnicity, country of birth, marital status, geographic region, and urbanicity were all significantly associated with socioeconomic status as measured by both household income and educational attainment. Of the sampled population, 66.9% were CMV-seropositive. CMV seropositive participants were significantly more likely to have a lower income and lower educational attainment than their seronegative counterparts. Compared to seronegative participants, seropositive participants were also significantly older, more likely to be female than male, and less likely to be non-Hispanic White or born in the US.
Table 1.
Baseline Characteristics of the Study Population by Household Income, Educational Attainment, and Cytomegalovirus Serostatus, National Health and Nutrition Examination Survey III, 1988–1994.
| Overall | Household income a
|
Educational attainment
|
CMV serostatus
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | Low-mid | Middle | High | <HS | HS | >HS | Positive | Negative | ||
| Household income, % c,d | ||||||||||
| Low | 17.0 | – | – | – | – | 53.5 | 30.8 | 15.6 | 81.6 | 18.4 |
| Low-Middle | 11.2 | – | – | – | – | 42.0 | 32.4 | 25.6 | 75.9 | 24.1 |
| Middle | 33.7 | – | – | – | – | 22.7 | 40.6 | 36.7 | 67.4 | 32.6 |
| High | 38.1 | – | – | – | – | 7.5 | 29.1 | 63.4 | 55.0 | 45.0 |
| Educational attainment, % b,d | ||||||||||
| < High school | 25.3 | 37.5 | 19.3 | 31.5 | 11.7 | – | – | – | 84.2 | 15.8 |
| High school | 33.4 | 15.6 | 10.7 | 40.7 | 33.0 | – | – | – | 69.1 | 30.9 |
| > High school | 41.3 | 6.3 | 6.8 | 29.4 | 57.4 | – | – | – | 54.6 | 45.4 |
| Age, % b,c,d | ||||||||||
| 25–39 years | 39.6 | 18.4 | 11.7 | 36.2 | 33.8 | 18.2 | 35.3 | 46.6 | 53.2 | 46.8 |
| 40–59 years | 35.0 | 13.2 | 7.7 | 30.3 | 48.8 | 21.6 | 33.6 | 44.8 | 68.2 | 31.8 |
| 60+ years | 25.4 | 20.3 | 15.4 | 34.4 | 30.0 | 41.6 | 30.4 | 28.0 | 86.2 | 13.8 |
| Gender, % b,c | ||||||||||
| Female | 52.5 | 19.3 | 11.6 | 33.1 | 36.0 | 24.8 | 36.4 | 38.8 | 72.0 | 28.0 |
| Male | 47.5 | 14.5 | 19.7 | 34.3 | 40.5 | 25.9 | 30.1 | 44.0 | 61.4 | 38.6 |
| Race/ethnicity, % b,c,d | ||||||||||
| Non-Hispanic White | 77.4 | 11.8 | 10.0 | 34.4 | 43.8 | 20.9 | 34.5 | 44.5 | 61.0 | 39.0 |
| Non-Hispanic Black | 10.6 | 37.0 | 15.2 | 29.4 | 18.4 | 33.8 | 36.5 | 29.7 | 86.6 | 13.4 |
| Mexican-American | 4.6 | 46.1 | 16.5 | 22.9 | 14.5 | 59.8 | 22.1 | 18.1 | 88.9 | 11.1 |
| Other | 7.4 | 27.8 | 15.2 | 38.2 | 18.8 | 38.1 | 24.2 | 37.7 | 88.2 | 11.8 |
| Country of birth, % b,c,d | ||||||||||
| US | 86.6 | 15.3 | 10.6 | 34.0 | 40.0 | 23.0 | 35.2 | 41.8 | 63.4 | 36.6 |
| Other | 13.4 | 29.9 | 15.0 | 30.9 | 25.1 | 40.8 | 21.5 | 37.8 | 89.4 | 10.6 |
| Marital status, % b,c,d | ||||||||||
| Married/cohabitating | 69.5 | 12.3 | 9.6 | 35.0 | 43.1 | 23.0 | 34.7 | 42.3 | 64.9 | 35.1 |
| Widowed/separated/divorced | 19.8 | 31.9 | 16.0 | 29.7 | 22.4 | 35.5 | 32.8 | 31.7 | 78.4 | 21.6 |
| Never married | 10.7 | 21.3 | 12.7 | 32.3 | 33.7 | 21.4 | 26.3 | 52.4 | 58.7 | 41.3 |
| Geographical region, % b,c,d | ||||||||||
| Northeast | 20.7 | 15.4 | 12.4 | 33.6 | 38.6 | 24.8 | 32.2 | 43.1 | 59.3 | 40.7 |
| Midwest | 24.2 | 15.1 | 11.2 | 37.1 | 36.7 | 21.8 | 37.5 | 40.7 | 62.2 | 37.8 |
| South | 33.7 | 20.8 | 12.0 | 33.6 | 33.6 | 30.6 | 34.6 | 34.8 | 74.8 | 25.2 |
| West | 21.4 | 14.8 | 8.7 | 30.0 | 46.5 | 21.6 | 28.1 | 50.2 | 67.2 | 32.8 |
| Urbanicity, % b,c | ||||||||||
| Rural | 48.3 | 14.7 | 9.1 | 30.7 | 45.6 | 21.1 | 30.0 | 48.9 | 67.1 | 32.9 |
| Urban | 51.7 | 19.2 | 13.1 | 36.5 | 31.2 | 29.2 | 36.6 | 34.2 | 66.8 | 33.2 |
| CMV serostatus, % b,c | ||||||||||
| Seropositive | 66.9 | 20.8 | 12.8 | 34.5 | 31.9 | 31.6 | 34.5 | 33.9 | – | – |
| Seronegative | 33.1 | 9.1 | 7.9 | 32.4 | 50.6 | 12.0 | 31.2 | 56.9 | – | – |
Household income level was measured by the income-to-poverty ratio, calculated by dividing total family income by the annual poverty threshold on the basis of household size and categorized as low (≤1.30), low-middle (> 1.30 to ≤1.85), middle (> 1.85 to ≤3.50), and high (> 3.50).
Comparison of distributions between income categories statistically significant (P value <0.05).
Comparison of distributions between education categories statistically significant (P value <0.05).
Comparison of distributions by CMV serostatus statistically significant (P value <0.05).
3.2. Overall associations with all-cause mortality
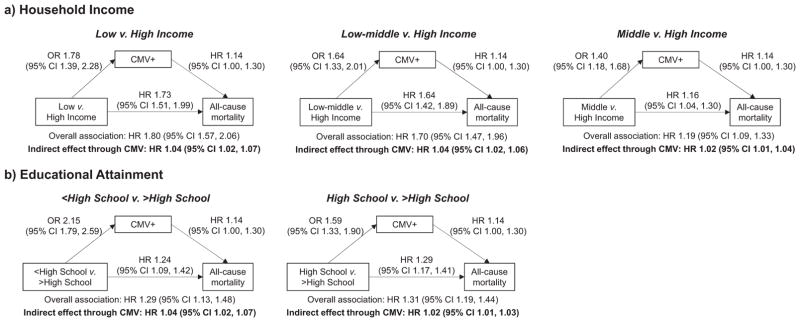
Fig. 2 shows the cumulative probability of all-cause mortality stratified by household income (Fig. 2a), educational attainment (Fig. 2b), and CMV serostatus (Fig. 2c). We observed a higher cumulative probability of mortality among low SES individuals as well as among CMV seropositive individuals. The covariate adjusted hazard ratios for the overall all-cause mortality associations are shown in Fig. 3. Adjusting for covariates, the hazard ratios for the overall association between household income and all-cause mortality were statistically significant (see Fig. 3a): compared to high income individuals, the HR’s were 1.80 (95% CI: 1.57, 2.06) for low income individuals, 1.70 (95% CI: 1.47, 1.96) for low-middle income individuals, and 1.19 (95% CI: 1.06, 1.33) for middle income individuals. Those with less than a high school education (HR 1.29; 95% CI: 1.13, 1.48) or a high school education (HR 1.31; 95% CI: 1.19, 1.44) had significantly higher mortality than those who had attained more than a high school education (see Fig. 3b). The all-cause mortality HR comparing CMV seropositive individuals to seronegative individuals was 1.14 (95% CI: 1.00, 1.30), which was only marginally significant with a P value of 0.06.
Fig. 2.
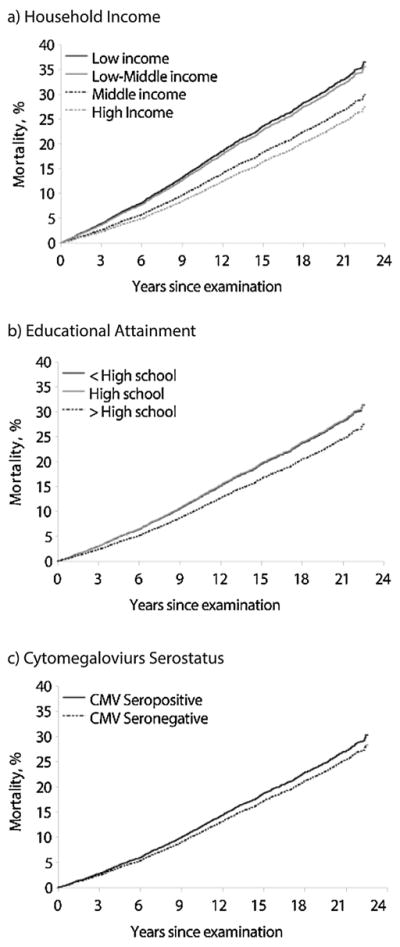
a–c. Covariate-adjusted Kaplan-Meier Failure Curves for All-cause Mortality Plotted by Household Income, Educational Attainment, and Cytomegalovirus (CMV) Seropositivity, National Health and Nutrition Examination Survey III, 1988–1994.
Fig. 3.
a–b. Associations Between Socioeconomic Status (Household Income Level and Educational Attainment), CMV Seropositivity, and All-cause Mortality Among US Adults ≥25 Years of Age, National Health and Nutrition Examination Survey III, 1988–1994.
3.3. Indirect effects
Fig. 3 also shows the decomposition of the overall association between the SES variables and all-cause mortality into the indirect effect mediated through CMV seropositivity and a second component representing the effect of SES through all other pathways. We found modest, but statistically significant indirect effects of household income on all-cause mortality when comparing low to high income individuals (indirect effect HR 1.04; 95% CI: 1.02, 1.07), low-middle to high income individuals (indirect effect HR 1.04; 95% CI: 1.02, 1.06), and middle to high income individuals (indirect effect HR 1.02; 95% CI: 1.01, 1.04) (Fig. 3a). The indirect effects through CMV seropositivity accounted for 6% (95% CI: 3%, 12%) of the overall mortality association for low compared to high income individuals, 7% (95% CI: 3%, 13%) for low-middle versus high income individuals, and 11% (95% CI: 5%, 43%) for middle versus high income individuals.
Similar results were found for educational attainment, with statistically significant indirect effect HR’s of 1.04 (95% CI: 1.02, 1.07) comparing those with less than a high school education to those with greater than a high school education and of 1.02 (95% CI: 1.01, 1.03) comparing those with a high school education to those with greater than a high school education (see Fig. 3b). These indirect effects accounted for 15% (95% CI: 7%, 32%) and 7% (95% CI: 3%, 16%) of the overall education-mortality associations, respectively.
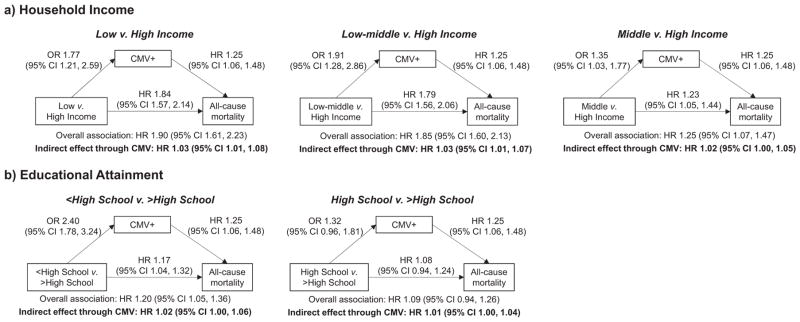
3.4. Effect modification by age
Fig. 4 shows the results of the mediation analysis for individuals who were at least 60 years of age (Supplemental Fig. 1 and Supplemental Fig. 2 shows the age-stratified results for all age groups). Age modified several of the pathways linking SES, CMV seropositivity, and all-cause mortality. Neither the overall association between household income and mortality, nor the direct effect of income on mortality not mediated through CMV were statistically significant in the youngest age group. Moreover, while the associations between low household income and CMV seropositivity were observed across all age groups, the association between CMV seropositivity and increased mortality did not emerge until middle age and was only statistically significant for those at least 60 years of age. Likewise, the indirect effect of household income on all-cause mortality mediated through CMV was only statistically significant for those in the oldest age group, which accounted for 5% (95% CI: 1%, 12%) of the overall income-mortality association for low compared to high income individuals, 5% (95% CI: 1%, 12%) for low-middle versus high income individuals, and 9% (95% CI: 2%, 28%) for middle versus high income individuals.
Fig. 4.
a–b. Associations Between Socioeconomic Status (Household Income Level and Educational Attainment), CMV Seropositivity, and All-cause Mortality Among US Adults ≥60 Years of Age, National Health and Nutrition Examination Survey III, 1988–1994.
In contrast to what we observed for household income, the overall association between educational attainment and all-cause mortality, as well as the direct effect not mediated through CMV became weaker with increasing age. Although the associations between educational attainment and CMV seropositivity were strong and statistically significant across age groups, none of the indirect effects of educational attainment on all-cause mortality mediated through CMV were statistically significant.
3.5. Sensitivity analysis
The associations between CMV seropositivity and selected behavioral and clinical characteristics, potential confounders of the CMV-mortality association, overall and stratified by age, are shown in Supplemental Table 1. No statistically significant associations between CMV seropositivity and cigarette use, alcohol use, Charlson index, or BMI were detected in the overall analysis. In the age-stratified analyses, there was a statistically significant association between CMV seropositivity and a higher Charlson index only in the oldest age group.
4. Discussion
In the present study, we quantified the extent to which CMV infection contributes to socioeconomic disparities in all-cause mortality in a nationally representative sample of US adults and assessed whether the pathways linking SES, CMV, and mortality were modified by age. Consistent with prior studies conducted in the NHANES III cohort (Suresh et al., 2011; Rask et al., 2009; Sabanayagam and Shankar, 2012), we found strong and statistically significant associations between low socioeconomic status and increased mortality in the overall study population. Indeed, individuals in the lowest income group were 1.8 times as likely to die within the follow-up period compared to those in the highest income group and individuals with at most a high school education were approximately 1.3 times as likely to die within the follow-up period compared to those who had attained more than a high school education. CMV infection, which affected over 65% of individuals in our sample, appeared to account for approximately 6–15% of these associations. Taken together, our findings suggest that interventions targeting the pathways linking SES, CMV, and mortality may reduce persistent socioeconomic disparities in mortality at the population level.
To the best of our knowledge, this is the first study to quantify the extent of the association between SES and mortality that is attributable to infection with CMV. However, our study builds on prior research that has shown an association between SES and CMV (Bate et al., 2010; Cannon et al., 2010; Colugnati et al., 2007; Dowd et al., 2009a; Dowd et al., 2009b; Staras et al., 2006; Simanek et al., 2009), and between CMV and mortality (Gkrania-Klotsas et al., 2013; Roberts et al., 2010; Simanek et al., 2011). Notably, several studies conducted in US population-representative samples (including the NHANES III cohort) have shown significant socioeconomic disparities in CMV seroprevalence persisting across age groups, with lower income and education groups having a higher seroprevalence (Bate et al., 2010; Cannon et al., 2010; Dowd et al., 2009a; Staras et al., 2006; Simanek et al., 2009). These socioeconomic gradients have been seen in children as well, with those growing up in lower SES households having a higher burden of infection compared to those who grow up in higher SES households (Dowd et al., 2009b). In our study, we observed strong and statistically significant associations between SES and CMV seropositivity regardless of the measure assessed (household income or educational attainment) and in all age groups.
Although the association between CMV seropositivity and all-cause mortality in our overall sample was only marginally significant (P value = 0.06), the magnitude of the association (HR: 1.14; 95% CI: 1:00, 1.30) was similar to statistically significant associations that have been demonstrated in prior population-based studies (Gkrania-Klotsas et al., 2013; Roberts et al., 2010; Simanek et al., 2011). For example, drawing from the same NHANES III sample as analyzed in the present study, Simanek et al. found that CMV seropositivity was significantly associated with higher all-cause mortality (HR 1.19, 95% CI: 1.01, 1.41) (Simanek et al., 2011). The small differences in the CMV-mortality association observed in this earlier study compared to the present study is likely attributable to the shorter follow-up period, since at the time mortality follow-up was only available through 2006, as well as slightly different covariate adjustment. The NHANES III findings are also consistent with a study by Gkrania-Klotsas et al. that was conducted in a European sample of adults 40–79 years of age, which found that the hazard of all-cause mortality among those seropositive for CMV was 1.16 (95% CI: 1.07,1.26) times the hazard of mortality compared to those seronegative for CMV (Gkrania-Klotsas et al., 2013).
Our age-stratified analyses revealed that the associations between SES, CMV seropositivity, and mortality may be modified by age. Indeed, while the association between CMV seropositivity and all-cause mortality was only slightly above the null and non-significant in the youngest age group (HR 1.08; 95% CI: 0.64, 1.82), a much stronger association was evident among those 40–59 years of age (HR 1.38; 95% CI: 0.99, 1.93) and those 60 years of age and older (HR 1.26; 95% CI: 1.06, 1.48), with statistical significance only observed in the oldest age group. This is consistent with evidence suggesting that the immune system’s ability to keep CMV in a quiescent state may wane over the life course. Along these lines, a growing body of evidence suggests that CMV is a primary contributor to altered immune signatures considered characteristic of aging, as clonal expansion of CMV-specific memory T-cells are triggered in response to subclinical CMV reactivation over time (Derhovanessian et al., 2011; Hadrup et al., 2006; Khan et al., 2002; Pawelec, 2013; Pawelec and Derhovanessian, 2011). This is illustrated by the near universal marked accumulation of these late-stage differentiated potentially “senescent” CD8+ T cells seen in the majority of CMV-seropositive but not seronegative individuals (Derhovanessian et al., 2010), which is a major component of the “Immune Risk Profile” associated with 2, 4 and 6-year mortality in the Swedish OCTO/NONA longitudinal studies (Pawelec et al., 2009).
Not surprisingly, CMV seropositivity in our sample showed a strong age gradient, ranging from 53% among individuals 25–39 years of age to 86% among individuals 60 years of age and older. This is likely attributable to an increased cumulative probability of exposure to the virus over the life course, as the prevalence of CMV in the US does not appear to be declining over calendar time (Bate et al., 2010) and thus a strong birth-cohort effect is unlikely. Why some individuals remain uninfected to the virus in old age despite a lifetime possibility of exposure remains unknown. One possibility is that these individuals display a unique biological resilience to infection with the virus, and that this resilience is likewise protective against other health conditions and associated mortality. Although there may be intrinsic variation in biological susceptibility to infection in the population due to genetic factors (Sansoni et al., 2014), our age-stratified analyses showed that a strong association between low SES and increased prevalence of CMV remained even in old age, suggesting that there are indeed extrinsic factors shaping risk for infection even in old age.
Along these lines, there may likewise be extrinsic factors related to SES that modulate the aging immune system’s ability to keep CMV in a quiescent state. For example, low SES may be associated with reduced intake of micronutrients such as zinc and selenium that are thought to be critical to maintaining immune cells (Mocchegiani et al., 2014), and particularly natural killer and natural killer T cells (Mocchegiani et al., 2009),and that likely affect how well the body is able to control the pathogen (Tyznik et al., 2014). Furthermore, given strong evidence suggesting that CMV reactivation is triggered by psychosocial stress (Caserta et al., 2008; Rector et al., 2014; Sarid et al., 2002) and persons of low SES are more likely to be exposed to stressors compared to higher SES individuals (Kristenson et al., 2004), it is likely that the mechanisms by which CMV impacts socioeconomic disparities in mortality also include pathways related to increased subclinical reactivation of the virus over the life course. Indeed, several studies have shown that low SES is associated with increased levels of CMV-specific IgG antibodies, a marker of viral reactivation (Dowd et al., 2008; Dowd and Aiello, 2009; Dowd et al., 2012). Unfortunately, CMV IgG antibody levels were only available for females <50 years of age and no other biomarkers of reactivation were available in NHANES III, and thus the CMV mediating pathway assessed in the present study incorporates the mechanisms related to both CMV seropositivity and reactivation. To further inform intervention efforts aimed at reducing SES disparities in mortality related to CMV infection, future studies should attempt to tease out which portion of the mediating effect is due to seropositivity compared to the portion that is attributable to reactivation.
We also observed that age modified the overall associations between SES and all-cause mortality, with the nature of the modification depending on the SES metric assessed. While low income was more strongly associated with increased mortality in the oldest age groups, educational attainment was more strongly associated with mortality in the youngest age group. Thus, income may be a better metric for capturing socioeconomic disparities in mortality among older individuals, while education may be a more sensitive metric in younger populations. These differences may also be attributable to temporal changes in the relative meaning of education in relation to socioeconomic trajectories (National Research Counscil Panel on Race, 2004) or to a birth-cohort effect related to widening educational disparities in mortality over time (Hummer and Hernandez, 2013).
An important strength of our study is that we employed the new IORW technique to assess mediation (Nguyen et al., 2015), which overcomes many of the limitations of traditional approaches that assess mediation through standard adjustment methods (i.e., comparison of estimates from models with and without adjustment for the mediator). However (Schisterman et al., 2009; Lange and Hansen, 2011), like all mediation analyses, the validity of our results also hinge on the assumption of no confounding after adjusting for measured covariates with respect to the effects of (1) the exposure on the mediator, (2) the exposure on the outcome, and (3) the mediator on the outcome, and that (4) none of the confounders of the effect of the mediator on the outcome are affected by the exposure (Nguyen et al., 2015).
Trajectories of socioeconomic status, and particularly educational attainment, are frequently set relatively early in the life course. Another simplifying assumption we make in the interpretation of our results is that CMV infection, which is generally acquired in childhood or adolescence (Harrison, 2014), does not itself cause lower educational attainment and income. Indeed, there is some evidence that CMV infection and reactivation may play a role in cognitive decline in the elderly (Aiello et al., 2006; Barnes et al., 2015), pointing to a potential mechanism by which CMV could impact SES. A prior cross-sectional study based on the NHANES III study population did not, however, support an association between CMV seropositivity and cognitive function in children (Tarter et al., 2014). Future longitudinal studies may, however, be warranted to further elucidate the potential bi-directional relationship between SES and CMV infection across the life course in relation to mortality.
The focus of the present analysis was to examine the extent to which CMV mediates the association between SES and all-cause mortality. However, future studies disentangled by cause of death may provide additional information about how CMV contributes to socioeconomic disparities in mortality. Indeed, prior studies have implicated CMV specifically in CVD-related mortality (Simanek et al., 2011; Savva et al., 2013) and some evidence suggests that the virus may contribute to cancer-related deaths as well (Barami, 2010; Herbein and Kumar, 2014; Michaelis et al., 2009; Soderberg-Naucler, 2006). Among the elderly, it may also be informative to examine the association between CMV and deaths due to accidents, as prior evidence suggests that CMV may play an important role in frailty (Wang et al., 2010). Furthermore, to our knowledge, no studies have specifically examined the role of other prevalent latent infections, such as herpes simplex virus (HSV)-1 and Helicobacter pylori, in mediating the SES-mortality association in the general US population. Although some evidence suggests that infection with CMV, but not other persistent herpesviruses, influences immune aging36 and prior studies have not found an association between HSV-1 or H. pylori seropositivity with mortality (Chen et al., 2013; Simanek et al., 2015), the extent to which other persistent pathogens contribute to SES disparities in mortality may warrant further investigation.
Overall, our findings suggest that CMV may partially contribute to socioeconomic disparities in all-cause mortality among adults in the United States. We observed modest, but statistically significant indirect effects of SES on all-cause mortality mediated through CMV. Two components affect the magnitude of the indirect effect estimates, including (1) the association between SES and CMV and (2) the association between CMV and mortality. Our results suggested that the SES-CMV association was a much stronger contributor to the indirect effects than the CMV-mortality association, and thus interventions that target the former pathway may have a greater impact on reducing socioeconomic disparities in mortality mediated through CMV. However, as CMV is a highly prevalent virus (Staras et al., 2006), neither pathway is negligible with regard to its potential contribution to socioeconomic disparities in mortality. As CMV is generally acquired in the early life course (Harrison, 2014), it may also be more feasible to target the CMV-mortality pathway, for example by addressing psychosocial stressors that may contribute to CMV reactivation over the life course. Prophylactic vaccination could also be considered, although thus far there are few candidate vaccines (Pass et al., 2009). Moreover, although the indirect effects of SES on mortality mediated through CMV did not vary substantially with age, the individual components comprising the indirect effects (i.e., the SES-CMV and the CMV-mortality associations) were modified by age, with CMV more strongly associated with mortality in older individuals. Thus, the mechanisms by which CMV contributes to socioeconomic disparities in all-cause mortality are likely intertwined with the mechanism underlying human aging and future interventions for reducing socioeconomic disparities related to CMV should be designed to address specific risk factors that may change over the life course.
Supplementary Material
Acknowledgments
Lydia Feinstein was supported by the Population Research Training grant (T32 HD007168) awarded to the Carolina Population Center at The University of North Carolina at Chapel Hill by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Lydia Feinstein and Allison Aiello were supported by the Population Research Infrastructure Program (P2C HD050924) awarded to the Carolina Population Center at The University of North Carolina at Chapel Hill by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Graham Pawelec is supported by the Croeni Foundation (http://croeni.org/g/g2015/) and by the European Commission under Grant Agreement FP7 259679, Integrated research on developmental determinants of aging and longevity, “IDEAL”.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.mad.2016.06.001.
Contributor Information
Lydia Feinstein, Email: lfeinst@email.unc.edu.
Christian E Douglas, Email: cedougla@email.unc.edu.
Rebecca C Stebbins, Email: rebecca7@email.unc.edu.
Graham Pawelec, Email: graham.pawelec@uni-tuebingen.de.
Amanda M. Simanek, Email: simaneka@uwm.edu.
Allison E Aiello, Email: aaiello@email.unc.edu.
References
- Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff. 2002;21(2):60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54(7):1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- Plan and operation of the Third National Health and Nutrition Examination Survey 1988–94. Vital Health Stat. 1994;1:32. [PubMed] [Google Scholar]
- Barami K. Oncomodulatory mechanisms of human cytomegalovirus in gliomas. J Clin Neurosci. 2010;17(7):819–823. doi: 10.1016/j.jocn.2009.10.040. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Capuano AW, Aiello AE, et al. Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J Infect Dis. 2015;211(2):230–237. doi: 10.1093/infdis/jiu437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund PA. Getting the Most out of the SAS® Survey Procedures: Repeated Replication Methods, Subpopulation Analysis, and Missing Data Options in SAS® v9.2 2009 [Google Scholar]
- Binder DA. On the variances of asymptotically normal estimators from complex surveys. Int Stat Rev/Revue Int Stat. 1983;51(3):279–292. [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(Suppl 1):S186–196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow NE. Discussion of professor cox’s paper. J R Stat Soc Ser B. 1972;34:216–217. [Google Scholar]
- Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- Caserta MT, O’Connor TG, Wyman PA, et al. The associations between psychosocial stress and the frequency of illness, and innate and adaptive immune function in children. Brain Behav Immun. 2008;22(6):933–940. doi: 10.1016/j.bbi.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Census Bureau, U.S. [accessed. 25.08.15];Current Population Survey Data on Educational Attainment. 2013 http://www.census.gov/hhes/socdemo/education/data/cps/index.html.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chen Y, Segers S, Blaser MJ. Association between Helicobacter pylori and mortality in the NHANES III study. Gut. 2013;62(9):1262–1269. doi: 10.1136/gutjnl-2012-303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty R, Stepner M, Abraham S, et al. The association between income and life expectancy in the United States, 2001–2014. JAMA. 2016;2016 doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNavas-Walt C, Proctor BD. Income and Poverty in the United States 2013 Current Population Reports. U.S. Census Bureau; Washington, DC: 2014. [Google Scholar]
- Deaton A. On death and money: history facts, and explanations. JAMA. 2016;2016 doi: 10.1001/jama.2016.4072. [DOI] [PubMed] [Google Scholar]
- Derhovanessian E, Maier AB, Beck R, et al. Hallmark features of immunosenescence are absent in familial longevity. J Immunol. 2010;185(8):4618–4624. doi: 10.4049/jimmunol.1001629. [DOI] [PubMed] [Google Scholar]
- Derhovanessian E, Maier AB, Hahnel K, et al. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol. 2011;92(Pt 12):2746–2756. doi: 10.1099/vir.0.036004-0. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Aiello AE. Socioeconomic differentials in immune response. Epidemiology. 2009;20(6):902–908. doi: 10.1097/EDE.0b013e3181bb5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Haan MN, Blythe L, Moore K, Aiello AE. Socioeconomic gradients in immune response to latent infection. Am J Epidemiol. 2008;167(1):112–120. doi: 10.1093/aje/kwm247. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009a;137(1):58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: burden of infection, health, and socioeconomic status in U.S. children. Soc Sci Med. 2009b;68(4):699–707. doi: 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Albright J, Raghunathan TE, Schoeni RF, Leclere F, Kaplan GA. Deeper and wider: income and mortality in the USA over three decades. Int J Epidemiol. 2011;40(1):183–188. doi: 10.1093/ije/dyq189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Palermo TM, Aiello AE. Family poverty is associated with cytomegalovirus antibody titers in U.S. children. Health Psychol. 2012;31(1):5–10. doi: 10.1037/a0025337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Jojic V, Sharma S, et al. Cytomegalovirus infection enhances the immune response to influenza. Sci Trans Med. 2015;7(281):281ra243. doi: 10.1126/scitranslmed.aaa2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Bound J, Colen CG. Excess black mortality in the United States and in selected black and white high-poverty areas, 1980–2000. Am J Public Health. 2011;101(4):720–729. doi: 10.2105/AJPH.2010.195537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkrania-Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ. Seropositivity and higher immunoglobuling antibody levels against cytomegalovirus are associated with mortality in the population-based European prospective investigation of Cancer-Norfolk cohort. Clin Infect Dis. 2013;56(10):1421–1427. doi: 10.1093/cid/cit083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- Hadrup SR, Strindhall J, Køllgaard T, et al. J Immunol. 4. Vol. 176. Baltimore, Md: 2006. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly; pp. 2645–2653. 1950. [DOI] [PubMed] [Google Scholar]
- Harkins L, Volk AL, Samanta M, et al. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet. 2002;360(9345):1557–1563. doi: 10.1016/S0140-6736(02)11524-8. [DOI] [PubMed] [Google Scholar]
- Harrison G. Cytomegalovirus. In: Cherry J, Demmler-Harrison GJ, Kaplan SL, Steinbach WJ, Hotez P, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Disease. 7. Elsevier Saunders; Philadelphia: 2014. p. 1969. [Google Scholar]
- Herbein G, Kumar A. The oncogenic potential of human cytomegalovirus and breast cancer. Front Oncol. 2014;4:230. doi: 10.3389/fonc.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874–875. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummer RA, Hernandez EM. The effect of educational attainment on adult mortality in the United States. Popul Bull. 2013;68(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiol Aging. 2004;25(5):619–627. doi: 10.1016/j.neurobiolaging.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Khan N, Shariff N, Cobbold M, et al. J Immunol. 4. Vol. 169. Baltimore, Md: 2002. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals; pp. 1984–1992. 1950. [DOI] [PubMed] [Google Scholar]
- Kristenson M, Eriksen HR, Sluiter JK, Starke D, Ursin H. Psychobiological mechanisms of socioeconomic differences in health. Soc Sci Med. 2004;58(8):1511–1522. doi: 10.1016/S0277-9536(03)00353-8. [DOI] [PubMed] [Google Scholar]
- Krueger PM, Tran MK, Hummer RA, Chang VW. Mortality attributable to low levels of education in the United States. PLoS One. 2015;10(7):e0131809. doi: 10.1371/journal.pone.0131809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T, Hansen JV. Direct and indirect effects in a survival context. Epidemiology. 2011;22(4):575–581. doi: 10.1097/EDE.0b013e31821c680c. [DOI] [PubMed] [Google Scholar]
- Liu R, Moroi M, Yamamoto M, et al. Presence and severity of Chlamydia pneumoniae and Cytomegalovirus infection in coronary plaques are associated with acute coronary syndromes. Int Heart J. 2006;47(4):511–519. doi: 10.1536/ihj.47.511. [DOI] [PubMed] [Google Scholar]
- Meyer PA, Penman-Aguilar A, Campbell VA, et al. Conclusion and future directions: CDC health disparities and inequalities report – United States, 2013: morbidity and mortality weekly report. Surveill Summ. 2013;62(Suppl 3):184–186. [PubMed] [Google Scholar]
- Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia. 2009;11(1):1–9. doi: 10.1593/neo.81178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Cipriano C, Malavolta M. NK and NKT cells in aging and longevity: role of zinc and metallothioneins. J Clin Immunol. 2009;29(4):416–425. doi: 10.1007/s10875-009-9298-4. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Costarelli L, Giacconi R, et al. Micronutrient-gene interactions related to inflammatory/immune response and antioxidant activity in ageing and inflammation. A systematic review. Mech Ageing Dev. 2014;136(-137):29–49. doi: 10.1016/j.mad.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Muennig P, Fiscella K, Tancredi D, Franks P. The relative health burden of selected social and behavioral risk factors in the United States: implications for policy. Am J Public Health. 2010;100(9):1758–1764. doi: 10.2105/AJPH.2009.165019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health. Hyattsville (MD): 2012. [PubMed] [Google Scholar]
- National Center for Health Statistics. Analytic and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988–94) Hyattsville, Maryland: Oct, 1996. [Google Scholar]
- National Center for Health Statistics. Analytic Guidelines for NCHS 2011 Linked Mortality Files. Hyattsville, Maryland: Sep, 2013. [Google Scholar]
- National Center for Health Statistics. Office of Analysis and Epidemiology, Public-use Linked Mortality File. 2015 http://www.cdc.gov/nchs/data_access/data_linkage/mortality.htm.
- National Research Counscil Panel on Race. Ethnicity, and health in later life. Race/Ethnicity, socioeconomic status, and health. In: Anderson N, Bulatao R, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. National Academies Press (US); Washington (DC): 2004. [PubMed] [Google Scholar]
- Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen EJ. Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. Am J Epidemiol. 2015;181(5):349–356. doi: 10.1093/aje/kwu278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ, Antonucci T, Berkman L, et al. Differences in life expectancy due to race and educational differences are widening, and many may not catch up. Health Aff. 2012;31(8):1803–1813. doi: 10.1377/hlthaff.2011.0746. [DOI] [PubMed] [Google Scholar]
- Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360(12):1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 2011;157(2):175–179. doi: 10.1016/j.virusres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19(1):47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Immunosenenescence: role of cytomegalovirus. Exp Gerontol. 2013 doi: 10.1016/j.exger.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Rao JNK, Scott AJ. On chi-Squared tests for multiway contingency tables with cell proportions estimated from survey data. Annal Stat. 1984;12(1):46–60. [Google Scholar]
- Rask K, O’Malley E, Druss B. Impact of socioeconomic, behavioral and clinical risk factors on mortality. J Public Health. 2009;31(2):231–238. doi: 10.1093/pubmed/fdp015. [DOI] [PubMed] [Google Scholar]
- Rector JL, Dowd JB, Loerbroks A, et al. Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain Behav Immun. 2014;38:133–141. doi: 10.1016/j.bbi.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172(4):363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabanayagam C, Shankar A. Income is a stronger predictor of mortality than education in a national sample of US adults. J Health Popul Nutr. 2012;30(1):82–86. doi: 10.3329/jhpn.v30i1.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta M, Harkins L, Klemm K, Britt WJ, Cobbs CS. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J Urol. 2003;170(3):998–1002. doi: 10.1097/01.ju.0000080263.46164.97. [DOI] [PubMed] [Google Scholar]
- Sansoni P, Vescovini R, Fagnoni FF, et al. New advances in CMV and immunosenescence. Exp Gerontol. 2014;55:54–62. doi: 10.1016/j.exger.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Sarid O, Anson O, Yaari A, Margalith M. Human cytomegalovirus salivary antibodies as related to stress. Clin Lab. 2002;48(5–6):297–305. [PubMed] [Google Scholar]
- Savva GM, Pachnio A, Kaul B, et al. Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell. 2013;12(3):381–387. doi: 10.1111/acel.12059. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53(5):747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- Simanek AM, Dowd JB, Aiello AE. Persistent pathogens linking socioeconomic position and cardiovascular disease in the US. Int J Epidemiol. 2009;38(3):775–787. doi: 10.1093/ije/dyn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One. 2011;6(2):e16103. doi: 10.1371/journal.pone.0016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanek AM, Cheng C, Yolken R, Uddin M, Galea S, Aiello AE. Herpesviruses, inflammatory markers and incident depression in a longitudinal study of Detroit residents. Psychoneuroendocrinology. 2014;50:139–148. doi: 10.1016/j.psyneuen.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanek AM, Dowd JB, Zajacova A, Aiello AE. Unpacking the ‘black box’ of total pathogen burden: is number or type of pathogens most predictive of all-cause mortality in the United States? Epidemiol Infect. 2015;143(12):2624–2634. doi: 10.1017/S0950268814003215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg-Naucler C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006;259(3):219–246. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Sorlie PD, Nieto FJ, Adam E, Folsom AR, Shahar E, Massing M. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arch Intern Med. 2000;160(13):2027–2032. doi: 10.1001/archinte.160.13.2027. [DOI] [PubMed] [Google Scholar]
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43(9):1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- Suresh S, Sabanayagam C, Shankar A. Socioeconomic status, self-rated health, and mortality in a multiethnic sample of US adults. Journal of Epidemiology/Japan Epidemiological Association. 2011;21(5):337–345. doi: 10.2188/jea.JE20100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter KD, Simanek AM, Dowd JB, Aiello AE. Persistent viral pathogens and cognitive impairment across the life course in the third national health and nutrition examination survey. J Infect Dis. 2014;209(6):837–844. doi: 10.1093/infdis/jit616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JE, Campbell JP, Edwards KM, et al. Rudimentary signs of immunosenescence in Cytomegalovirus-seropositive healthy young adults. Age. 2014;36(1):287–297. doi: 10.1007/s11357-013-9557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyznik AJ, Verma S, Wang Q, Kronenberg M, Benedict CA. Distinct requirements for activation of NKT and NK cells during viral infection. J Immunol. 2014;192(8):3676–3685. doi: 10.4049/jimmunol.1300837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GC, Kao WH, Murakami P, et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171(10):1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.