Abstract
The eye lens is a transparent and avascular organ in the front of the eye that is responsible for focusing light onto the retina in order to transmit a clear image. A monolayer of epithelial cells covers the anterior hemisphere of the lens, and the bulk of the lens is made up of elongated and differentiated fiber cells. Lens fiber cells are very long and thin cells that are supported by sophisticated cytoskeletal networks, including actin filaments at cell junctions and the spectrin-actin network of the membrane skeleton. In this review, we highlight the proteins that regulate the diverse actin filament networks in the lens and discuss how these actin cytoskeletal structures assemble and function in epithelial and fiber cells. We then discuss methods that have been used to study actin in the lens and unanswered questions that can be addressed with novel techniques.
Introduction
The eye lens is a remarkable spheroid organ composed of highly organized fiber cells covered by an anterior monolayer of epithelial cells (Figure 1). The lens presents a unique opportunity to study cell migration, elongation, packing, differentiation and aging all within the same tissue. Life-long lens growth is facilitated by the proliferation and differentiation of equatorial epithelial cells into fiber cells (Bassnett and Winzenburger, 2003; Kuszak, 1995; Kuszak et al., 2004a; Piatigorsky, 1981), followed by coordinated migration, elongation and stabilization of fiber cells (Kuszak et al., 2004b; Lovicu and Robinson, 2004; Piatigorsky, 1981; Taylor et al., 1996). Fiber cell morphogenesis is supported by three cytoskeletal systems: microtubules, intermediate filaments and actin filaments (F-actin). Single and bundled microtubules, which are arranged along the long axis of lens fibers, have been suggested to be important for cell elongation and vesicular transport (Kuwabara, 1968; Lo et al., 2003; Piatigorsky, 1975). Beaded intermediate filaments comprised of specialized intermediate filament proteins, CP49 (phakinin, Bfsp2) and filensin (CP115, Bfsp1) (Alizadeh et al., 2003; FitzGerald, 2009), are needed for mechanical integrity (Fudge et al., 2011; Gokhin et al., 2012) and maintaining life-long lens transparency (Gokhin et al., 2012; Oka et al., 2008; Sandilands et al., 1995). Of the F-actin networks, the best understood is the spectrin-actin membrane skeleton, composed of actin filaments cross-linked by α2β2-spectrin, which is integral for fiber cell packing (Gokhin et al., 2012; More et al., 2001; Nowak et al., 2009; Nowak and Fowler, 2012) and mechanical stiffness (Gokhin et al., 2012). In this review, we focus on the organization, regulation and functions of F-actin networks and their associated actin-binding proteins in the lens (summarized in Tables 1 and 2). We also cover approaches for studying actin in the lens and discuss unanswered questions about actin’s role in the lens.
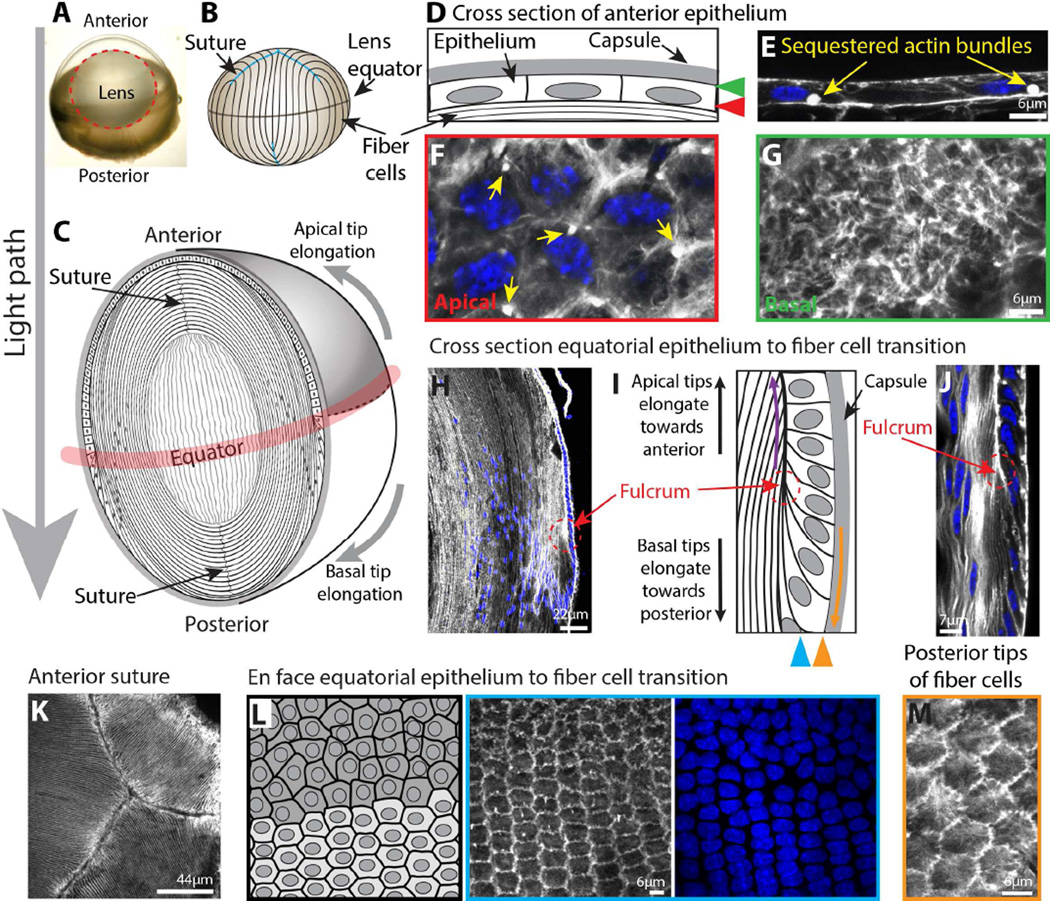
Figure 1. Actin cytoskeletal organization in the lens.
A) Dissected mouse eye; red dotted circle outlines lens in the intact eye. B) Diagram of mouse lens (not drawn to scale). The lens equator is demarcated by black horizontal line, and the sutures are indicated by blue lines. C) Diagram of lens anatomy showing anterior epithelial cells and bulk elongated fibers. Secondary fiber cells elongate toward the anterior and posterior poles (gray arrows) and meet at the anterior and posterior lens sutures. Lens equator indicated by red shading. D) Diagram of cuboidal anterior epithelial cells. Red arrowhead indicates apical surface shown in F (en face view), and green arrowhead indicates basal surface shown in G (en face view). E) Phalloidin (F-actin) and Hoechst (nuclei, blue) staining of anterior epithelial cells in cross section. F-actin is abundant at apical and basal surfaces of these cells and forms sequestered actin bundles (yellow arrows) near the apical surface. F and G) Phalloidin and Hoechst staining of flat-mounted anterior epithelial cells en face. On their apical surfaces, anterior lens epithelial cells have cortical actin fibers and sequestered actin bundles (F, yellow arrows). In contrast, on the basal surfaces of these cells, abundant actin stress fibers are observed (G). H) Low-magnification sagittal section of a mouse lens stained with phalloidin and Hoechst showing lens epithelial and fiber cell morphology near the lens equator. The lens fulcrum (red dotted circle and arrow) forms an anchorage point for the tips of many differentiating fiber cells as fiber cells rotate in orientation. I) Diagram of equatorial epithelial-to-fiber cell transition. Red arrows and circle indicate the lens fulcrum. Purple arrow pointing up and orange arrow pointing down show the directions of apical and basal tip migration, respectively, of elongating secondary fiber cells. Blue and orange triangles indicate en face focal plane in L and M, respectively. J) Phalloidin and Hoechst staining of the lens fulcrum (red dotted circle and arrow) showing elongation of newly formed secondary fiber cells. F-actin is enriched at the lens fulcrum and the apical junction between epithelial and secondary fiber cells. K) Phalloidin staining of the anterior suture of a mouse lens where tips of elongating fiber cells meet at the pole (en face view). F-actin is enriched at fiber cell tips. L) Diagram, and phalloidin and Hoechst staining of an en face view of equatorial epithelial cells. Equatorial epithelial cells rearrange from randomly packed cells into organized meridional rows of hexagonal cells. F-actin is disorganized in randomly packed equatorial epithelial cells and becomes localized to the cell membrane in hexagonal meridional cells. M) En face view of newly formed, phalloidin-stained secondary fiber cell posterior tips (basal-lateral side) on the capsule showing the perpendicular organization of the actin stress fibers with respect to the cell boundary.
Table 1.
Actin Binding Proteins in the Lens
| Protein | Known functions |
Epithelial Cell Localization (age, species) |
Fiber Cell Localization (age, species) |
Interactions With Lens Proteins |
Lens Phenotype of Knockouts, Mutants or Inhibitor Treatment |
References |
|---|---|---|---|---|---|---|
| α-actinin | Cross-links actin filaments in an anti-parallel orientation |
- | Vertices of cortical fiber cells (neonatal and adult rat) |
- | - | (Lo et al., 1997; Sjoblom et al., 2008) |
| Adducin | Caps actin filament barbed ends in actin- spectrin network |
- | Broad and short sides of fiber cells (adult, rat) |
- | - | (Fowler, 2013; Kaiser et al., 1989) |
| Arp2/3 complex |
Actin nucleation. Initiates branched actin filament assembly from preexisting filaments |
Cytoplasm (adult, mouse) |
Enriched at vertices of fiber cells (adult, mouse) |
N-cadherin and cortactin in fiber cells (not epithelial cells) |
- | (Amann and Pollard, 2001; Blanchoin et al., 2000; Leonard et al., 2011; Mullins et al., 1998) |
| Band 4.1 | Binds to and stabilizes actin- spectrin network |
Not present (embryo, chick) |
Short sides of fiber cells (embryo, chick) |
- | - | (Aster et al., 1986; Aster et al., 1984; Bagchi et al., 2004; Beebe et al., 2001; Fowler, 2013) |
| Band 4.9 (dematin) |
Bundles actin filaments |
- | Fiber cell membranes (adult, chick) |
- | - | (Faquin et al., 1988; Fowler, 2013) |
| Cofilin | Binds to actin filaments causing depolymerization (cofilin is inactivated by phosphorylation) |
- | - | - | Decreased phospho-cofilin and abnormal actin filament networks in RIP76/RALPB1 transgenic lenses; increased phospho-cofilin and abnormal secondary fiber cell migration and orientation in Rac1 conditional lens knockouts |
(Bamburg et al., 1980; Maddala et al., 2011a; Nishida et al., 1984; Sahu et al., 2014) |
| Cortactin | Bind and stabilizes branched actin filaments and activated (phosphorylated) cortactin recruits Arp2/3 to polymerize actin filaments |
Total and phospho- cortactin is enriched at vertices of hexagonal equatorial epithelial cells (adult, mouse) |
- | - | Loss of EphA2 leads to changes in actin and cortactin localization in hexagonal equatorial epithelial cells of meridional rows |
(Cheng et al., 2013; Uruno et al., 2001; Weaver et al., 2001) |
| Crystallin, α | Small heat shock proteins with chaperone-like functions |
Cytoplasm (adult, mouse) |
Cytoplasm (adult, mouse) |
- | Mutations in α- crystallins alter association with actin, a possible native substrate of α-crystallins |
(Andley et al., 2014; Bloemendal et al., 1984; Brown et al., 2007; Del Vecchio et al., 1984; Gopalakrishnan and Takemoto, 1992; Kibbelaar et al., 1979) |
| Crystallin, β/γ |
Structural proteins |
- | Cytoplasm (adult, mouse) |
- | Interacts with actin, and γ- crystallins may help stabilize actin filaments in mature fiber cells |
(Fan et al., 2012; Li et al., 2008; Rao et al., 2008) |
| Ezrin | In ERM/EPPD complexes that link actin filament networks with the plasma membrane |
- | Broad and short sides of fiber cells (adult, bovine/mouse); enriched on sides of fiber cells (embryo, chick) |
Aqp0, moesin, spectrin, plectin, periaxin, periplaxin, desmoyokin |
Genetic variation linked to human age-related cataracts |
(Bagchi et al., 2004; Bretscher, 1983; Lin et al., 2013; Maddala et al., 2011b; Straub et al., 2003; Wang and Schey, 2011) |
| Formin [isoform(s) unknown] |
Nucleates unbranched actin networks by promoting barbed end filament assembly |
Cytoplasm and nucleus (embryo, mouse) |
Cytoplasm and nucleus (embryo, mouse) |
- | Fiber cell degeneration |
(de la Pompa et al., 1995; Goode and Eck, 2007; Pruyne et al., 2002; Sagot et al., 2002) |
| Gelsolin | Severs actin filaments and caps actin filament barbed ends |
- | - | α-crystallins | - | (Andley et al., 2014; Yin and Stossel, 1980) |
| Myosin II | Bipolar filaments bind to anti- parallel actin filaments to generate filament sliding and contractility |
Apical surface of lens vesicle (embryo, mouse); cytoplasm and concentrated at apical junction between epithelial and fiber cells (embryo and neonatal, mouse) |
Cytoplasm (embryo and neonatal, mouse); basal membrane complexes (embryo, chick) |
- | Mutations in humans and mice cause cataracts; inhibitor treatment leads abnormal eye cup and lens vesicle formation and nuclear cataracts (mouse) as well as decreased lens stiffness and changes in focal length (chick) |
(Bassnett et al., 1999; Borges et al., 2011; Chauhan et al., 2009; De Rocco et al., 2013; Economou et al., 2012; Eiraku et al., 2011; Hao et al., 2012; Lang et al., 2014; Luck and Choh, 2011; Maddala et al., 2007; Plageman et al., 2011; Saposnik et al., 2014; Won et al., 2015; Zhang et al., 2012) |
| Plectin | Links actin and intermediate filaments to ERM/EPPD complexes |
- | Broad and short sides of fiber cell membranes (adult, bovine) |
Ezrin, moesin, periaxin, periplakin, desmoyokin, spectrin |
- | (Andra et al., 1998; Fontao et al., 2001; Jiu et al., 2015; Sandilands et al., 1995; Straub et al., 2003; Weitzer and Wiche, 1987) |
| α2β2-spectrin | Spectrin tetramers cross- link actin filaments to form an isotropic network at the membrane, the membrane skeleton |
- | Broad and short sides of fiber cells (adult, mouse) |
Ezrin, moesin, periaxin, periplaxin, desmoyokin, ankyrin-B |
- | (Cheng et al., 2015; Fowler, 2013; Gokhin et al., 2012; Nowak et al., 2009; Nowak and Fowler, 2012; Straub et al., 2003) |
| Tmod1 (mouse) Tmod4 (chicken) |
Caps actin filament pointed ends, inhibiting actin association and dissociation, promoting length regulation and stability |
Cytoplasm in differentiating equatorial epithelial cells (adult, mouse) |
Broad and short sides of fiber cells; not at vertices (adult, mouse) |
γTM (mouse), filensin (chick), spectrin, CP49 (indirect, mouse) |
Abnormal actin- spectrin network, disrupted fiber cell packing, decreased lens stiffness and gap junction coupling |
(Almenar-Queralt et al., 1999; Cheng et al., 2015; Fischer et al., 2003; Fischer et al., 2000; Fowler, 1996, 2013; Gokhin et al., 2012; Lee et al., 2000; Nowak et al., 2009; Nowak and Fowler, 2012; Sussman et al., 1996; Weber et al., 1994; Woo et al., 2000; Yamashiro et al., 2012) |
| Tropomyosin (γTM) |
Binds along sides of actin filaments to prevent severing and depolymerization |
Cytoplasm (adult, mouse) |
Broad and short sides of fiber cells (adult, mouse) |
Tmod1, Tmod4 | - | (Fischer et al., 2000; Fowler, 2013; Lee et al., 2000; Nowak et al., 2009; Woo and Fowler, 1994) |
Table 2.
Actin Regulating Proteins in the Lens
| Protein | Known functions |
Epithelial Cell Localization (age, species) |
Fiber Cell Localization (age, species) |
Interactions With Lens Proteins |
Lens Phenotype of Knockouts, Mutants or Inhibitor Treatment |
References |
|---|---|---|---|---|---|---|
| Abi-2 (c-abl- interactor) |
Adaptor that interacts with c- abl kinase to regulate WAVE- mediated Arp2/3 activation-driven actin assembly and dynamics |
Cytoplasm and enriched at the apical epithelial- fiber cell junction, especially near lens equator (embryo, mouse) |
Cytoplasm (embryo, mouse) |
Co-localizes with WAVE-2, cadherins and β- catenin at apical interface between epithelial and fiber cells |
Secondary fiber cell migration and orientation defects |
(Grove et al., 2004; Maddala et al., 2011) |
| Cdc42 | Filopodia formation |
Cytoplasm (neonatal, mouse) |
Cytoplasm, especially in the cortex at the basal and apical tips of fibers (neonatal, mouse) |
- | Loss of actin filopodia between the lens placode and developing retina leads to abnormal optic cup and lens pit shape |
(Chauhan et al., 2009; Chen et al., 2006; Chen et al., 2008) |
| p120-catenin | Binds cadherins to activate Rho GTPases to modulate actin dynamics |
- | - | - | Mislocalization of myosin IIB away from apical surface of lens placode leading to placode invagination defect |
(Lang et al., 2014) |
| Rac1 | Actin stress fiber formation |
Cytoplasm (neonatal, mouse) |
Cytoplasm (neonatal, mouse) |
- | Secondary fiber cell migration and orientation defects |
(Chen et al., 2006; Chen et al., 2008; Maddala et al., 2011) |
| Rap1 | Ras-like small GTPase needed for Cdc42 activation during cell-cell junction formation |
- | - | - | Incomplete elongation of primary fibers with gap between anterior tips of primary fibers and apical surface of anterior epithelium, decreased actin filaments and disrupted E- cadherin and β- catenin staining |
(Maddala et al., 2015) |
| RhoA/B/C | Promotes actin stress fiber formation |
Cytoplasm (RhoA, neonatal, mouse) |
Cytoplasm, especially in the cortex at the basal and apical tips of fibers (RhoA, neonatal, mouse) |
- | Transgenic lens expression of C3 Rho GTPase inhibitor leads to small lenses with swollen fibers and decreased actin filaments, adherens junctions, gap junction and water channels |
(Chen et al., 2006; Chen et al., 2008; Maddala et al., 2004; Rao et al., 2002) |
| RLIP76/RALBP 1 |
Effector of Ral1 and Rho/Rac |
- | - | - | Transgenic lens expression leads to small lenses and microphthalmia with abnormal actin filament networks, Cdc42 inactivation and lower levels of phospho-cofilin |
(Sahu et al., 2014) |
| Shroom3 | Recruits actin filaments via Mena-family of actin modulators that are needed for apical constriction |
- | - | Rock1/2 | Reduced actin filaments and myosin IIB at apical surface of lens placode leading to abnormal placode invagination |
(Plageman et al., 2010) |
| WAVE-2 | Activates Arp2/3 leading to actin polymerization |
Cytoplamic and enriched at the apical epithelial- fiber cell junction, especially near the lens equator (embryo, mouse) |
Cytoplasmic (embryo, mouse) |
- | - | (Maddala et al., 2011) |
1. Roles of actin in embryonic lens development and formation
Early lens development is characterized by invagination of a specialized region of the surface ectoderm (lens placode) to form the lens pit, which then pinches off from the overlying ectoderm to form the lens vesicle (Lovicu and Robinson, 2004; Piatigorsky, 1981). During formation of the optic cup (the presumptive retina and retinal pigmented epithelium) and invagination of the lens vesicle, actin-rich filopodia extend from the basal posterior surface of lens pit to the anterior surface of the retina, and contract to shape the embryonic eye (Chauhan et al., 2009; Mann, 1964; McAvoy, 1980). The Rho family of GTPases (RhoA, B and C; Rac 1, 2 and 3; Cdc42) is central to actin cytoskeleton regulation in epithelial morphogenesis, including cell adhesion, migration, filopodia extension, and apical domain contractions (Bishop and Hall, 2000; Heasman and Ridley, 2008; Linseman and Loucks, 2008; Nobes and Hall, 1994; Nobes and Hall, 1999; Villalonga and Ridley, 2006). Changes in Cdc42 cause a loss of actin-rich filopodia that leads to an abnormally shaped optic cup and lens pit, suggesting that F-actin filopodia transmit force to fine-tune embryonic eye morphogenesis (Chauhan et al., 2009). Three-dimensional culture of Rx+ (retinal homeobox gene) embryonic stem cells stimulates optic cup formation in vitro, and treatment of cultures with the myosin II ATPase inhibitor, blebbistatin, or the Rho-kinase (ROCK) inhibitor, Y-27632, blocks invagination of the optic cup (Eiraku et al., 2011). Lens pit filopodia also contain activated myosin II with phosphorylated myosin light chain (pMLC), and blebbistatin treatment to inhibit myosin II ATPase activity results in lengthening of these filopodia. In contrast, calyculin A treatment (inhibitor of myosin phosphatase, which leads to increased pMLC, thereby activating myosin II ATPase) results in shorter filopodia (Chauhan et al., 2009).
As the lens placode invaginates to form the lens vesicle, epithelial cells undergo apical constriction via contraction of an actomyosin network (Lang et al., 2014; Quintin et al., 2008), regulated by MLC phosphorylation and activation of myosin IIB at the apical surfaces of lens epithelial cells (Lang et al., 2014). Inhibition of myosin, actin dynamics or RhoA through specific inhibitors disrupts lens invagination (Borges et al., 2011; Plageman et al., 2011). Shroom3, a Rock1/2 and actin-binding cytoskeletal protein, is required for apical constriction of the lens placode, and loss of Shroom3 leads to reduced F-actin and myosin IIB at the apical surface of the lens placode (Plageman et al., 2010). Shroom3 likely recruits F-actin via the Mena-family of actin modulators that are known to play a role in apical constriction (Plageman et al., 2010; Roffers-Agarwal et al., 2008). Similar to the effects of Shroom3, loss of p120-catenin, a cadherin-binding protein that activates Rho GTPases to modulate actin dynamics (Pieters et al., 2012), also causes defects in lens invagination and leads to mislocalization of myosin IIB away from the apical surface (Lang et al., 2014). A new study shows that Cdc42 inhibits Shroom3-induced cell contraction in the lens placode to allow cell elongation, suggesting that a balance between these opposing forces on the actin cytoskeleton is needed for normal orientation and planar cell polarity in the lens placode (Muccioli et al., 2016). These studies indicate that contractility of the actomyosin network is important for early eye and lens formation.
In the lens vesicle, apical surfaces of epithelial cells are directed toward the lumen, and consequently, components of the basal lamina secreted by these cells surround the external surface of the vesicle, eventually creating a thin collageneous basement membrane (lens capsule) to encase the lens (Lovicu and Robinson, 2004). Posterior epithelial cells elongate at their apical ends into the vesicle lumen and differentiate into primary lens fiber cells to fill the bulk of the embryonic lens (Lovicu and Robinson, 2004). Rap1, a Ras-like small GTPase required for Cdc42 activation during cell-cell junction formation (Hogan et al., 2004), is needed for elongation of primary lens fibers and adhesion of fiber cell tips to the apical surface of the anterior epithelium (Maddala et al., 2015a). Rap1 also regulates E-cadherin at cell-cell junctions (Hogan et al., 2004), and E-cadherin junctions recruit cortactin and Arp2/3 to initiate F-actin assembly (Ehrlich et al., 2002; Helwani et al., 2004; Kovacs et al., 2002; Vasioukhin et al., 2000). Loss of Rap1 in the lens results in decreased F-actin and disrupts E-cadherin and β-catenin staining at the epithelial-fiber apical junction, leading to gaps between the apical surfaces of anterior epithelial cells and tips of elongating primary fiber cells (Maddala et al., 2015a). Transgenic lens expression of a Rho GTPase specific inhibitor C3-exoenzyme leads to small and very disrupted embryonic lenses and swollen fiber cells with decreased F-actin, adherens junctions and gap junctions (Maddala et al., 2004; Rao et al., 2002). Similarly, inhibition of Cdc42 activation due to transgenic lens expression of RLIP76/RALBP1, an effector of two Ras family GTPases, Ral and Rho/Rac (Jullien-Flores et al., 1995; Park and Weinberg, 1995), causes abnormal F-actin networks, small lenses and microphthalmia (Sahu et al., 2014).
2. Functions of actin in lens epithelial and fiber cell morphogenesis
2.1 Epithelial cells
The anterior hemisphere of the late embryonic and adult lens is covered by a monolayer of epithelial cells. Epithelial cells near the anterior pole are cuboidal in shape and quiescent. Basal surfaces of these epithelial cells contain actin stress fibers (Liou and Rafferty, 1988; Weber and Menko, 2006) (Figure 1G) and F-actin-rich lamellipodia (Weber and Menko, 2006) that are likely linked to the lens capsule through focal adhesions and integrin receptors (Schoenwaelder and Burridge, 1999). On apical and lateral surfaces, anterior epithelial cells (Bassnett, 2005) with adherens junctions (Zampighi et al., 2000) with adherens junctions (Weber and Menko, 2006) (Figure 1F). On their basal surfaces, actin stress fibers and lamellipodia likely aid the spreading of anterior epithelial cells on the lens capsule and may help to maintain the undifferentiated state as in other cell types (McBeath et al., 2004; Woods et al., 2005), while apical cortical actin stress fibers help stabilize the cuboidal cell shape (Weber and Menko, 2006). In addition to these typical actin structures, the apical domains of anterior epithelial cells also contain unusual polygonal arrays of F-actin associated with myosin II and sequestered actin bundles (SABs) (Rafferty, 1985; Rafferty and Scholz, 1984, 1985, 1989; Rafferty et al., 1990; Scholz and Rafferty, 1988). Polygonal arrays of F-actin have been found in mouse, rabbit, squirrel, monkey and human lens epithelial cells (Rafferty and Scholz, 1985, 1989; Rafferty et al., 1990; Scholz and Rafferty, 1988; Yeh et al., 1986). These geodesic domes of actin and myosin filaments are proposed to flatten in order to maintain epithelial cell integrity during lens accommodation, but this has not been experimentally tested (Rafferty and Scholz, 1985; Yeh et al., 1986). SABs (Figure 1E and 1F) are composed of a single, elongated bundle of F-actin that is attached to the apical plasma membrane at one end and interacts with a perinuclear network of intermediate filaments at the other end (Rafferty and Scholz, 1985). In mice, the presence and size of SABs are strain- and age-dependent (Liou and Rafferty, 1988; Rafferty and Scholz, 1989). The function of SABs in maintaining lens epithelial cell shapes or behaviors is unclear.
Life-long lens growth depends on the proliferation and differentiation of equatorial epithelial cells into secondary fiber cells (Lovicu and Robinson, 2004). Epithelial cells in the anterior-most region of the lens equator disassemble their F-actin stress fibers and only have dispersed F-actin on their basal surfaces, retaining cortical actin fibers at their apical surfaces (Weber and Menko, 2006). As equatorial epithelial cells proliferate and start to differentiate, they increase in height in the apical-basal dimension but decrease in diameter, thus maintaining their volume (Bassnett, 2005). Concomitantly, dispersed F-actin on their basal surfaces collects into bundles that radiate from the center of the cell toward the cell periphery, along with some cortical actin fibers (Weber and Menko, 2006). In the posterior-most region of the lens equator, epithelial cells undergo a remarkable morphogenesis to change from randomly packed cells into hexagonally packed cells arranged into organized meridional rows (Figure 1L) (Bassnett et al., 1999; Cheng et al., 2013; Wu et al., 2015). Cortical F-actin fibers appear on lateral membranes of these cells (Weber and Menko, 2006) and are likely important for stabilizing N-cadherin junctions between these differentiating cells (Ferreira-Cornwell et al., 2000; Leong et al., 2000). F-actin is also enriched at the vertices (tricellular junctions) of these hexagonal cells near their basal-lateral interface and at their apical tips (Cheng et al., 2013) to form the lens fulcrum (Figure 1H–J). The lens fulcrum (Sugiyama et al., 2009) or modiolus forms an anchor point where tips of many differentiating fiber cells are located as fiber cells rotate in orientation (Zampighi et al., 2000). Bidirectional signaling through EphA2 activates Src and cortactin, leading to the proper localization and accumulation of actin that is essential for hexagonal cell shape, organized packing of meridional rows and the formation of the lens fulcrum (Cheng et al., 2013) (Figure 1L).
2.2 Newly differentiating and elongating secondary fiber cells
Coordinated elongation and migration of differentiating fiber cells is a beautiful example of collective cell migration in tissues (Montell, 2008; Pocha and Montell, 2014). At the lens equator, newly formed fiber cells are hexagonally shaped in cross-section (Figure 2). Each fiber cell has 4 short sides and 2 broad sides and is contacted by 6 neighboring cells one at each of the 4 short sides, and at each of the 2 broad sides (1 cell from a more superficial layer and 1 cell from a deeper layer), so that each fiber cell is surrounded by 6 nearest neighbors, connected along their lateral membranes and by 6 tricellular junctions. This arrangement leads to the addition of new layers, or shells, of fiber cells (Kuszak, 1995; Kuszak et al., 1991; Kuszak et al., 2004a) on the lens periphery that surrounds previous generations of fiber cells (Figure 2D). Secondary fiber cells maintain hexagonal packing during elongation, as anterior tips of these cells migrate along the apical surfaces of epithelial cells, while posterior tips crawl along the lens capsule towards the anterior and posterior lens poles, respectively (Lovicu and Robinson, 2004). The tips of elongating fiber cells detach from the anterior epithelium or the posterior capsule at the poles and contact the tips of opposing fiber cells from the other side of the lens to form Y-sutures (Kuszak et al., 2004b) (Figure 1K). Differentiation of newly formed secondary lens fiber cells is accompanied by an increase in ratio of polymerized F-actin vs. monomeric globular G-actin (Ramaekers et al., 1981). Thus, newly formed fiber cells have an extensive cortical F-actin network along the entire lengths of their lateral membranes, and F-actin is enriched at the tricellular junctions (vertices) of hexagonally packed young fiber cells on their lateral surfaces (Leonard et al., 2011; Nowak et al., 2009). Treatment of primary cultures of chick lens epithelial cells with cytochalasin-D causes epithelial cells to disassemble F-actin stress fibers, initiate fiber cell differentiation and assemble cortical F-actin bundles (Weber and Menko, 2006), indicating that F-actin network rearrangement is necessary to drive fiber cell elongation and differentiation (Beebe and Cerrelli, 1989; Mousa and Trevithick, 1977; Weber and Menko, 2006).
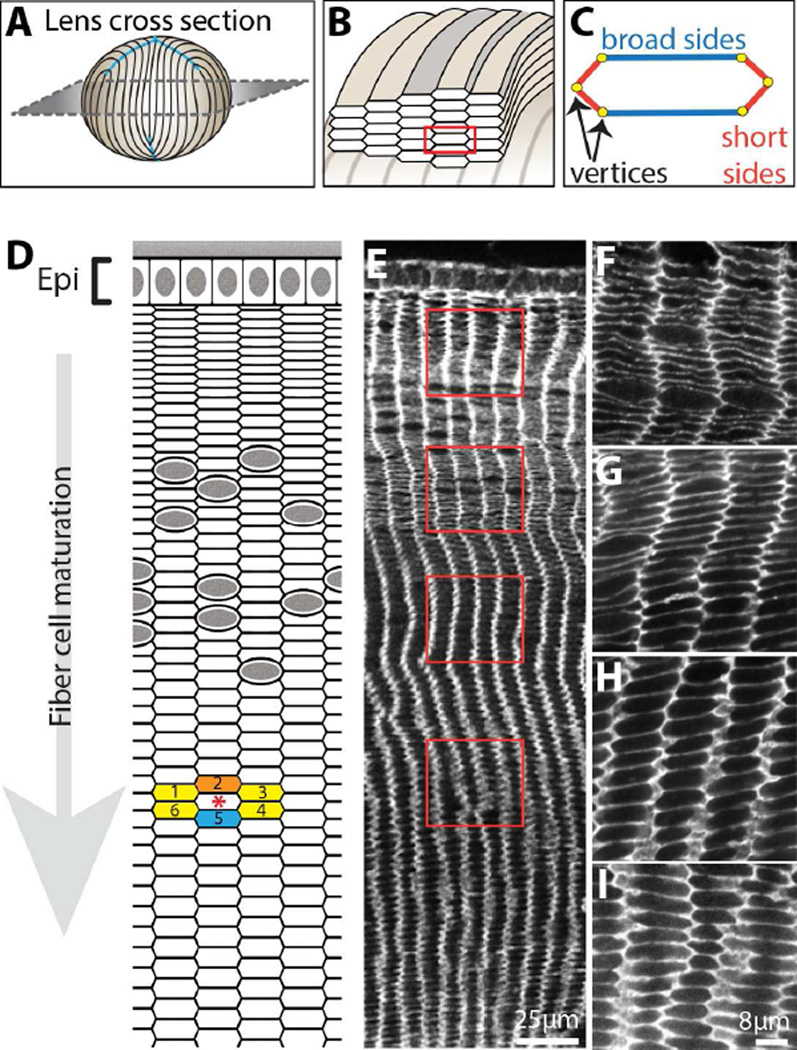
Figure 2. Actin cytoskeletal organization in lens fiber cells.
A) Diagram of a lens showing orientation of a cross section through the lens equator. B) Cut-away diagram of cross-sections of organized hexagonally packed fiber cells, rotated 90° with respect to A. An individual fiber cell is outlined with a red box. C) Diagram showing the broad sides (blue), short sides (red) and vertices (yellow) of an individual hexagonal fiber cell in cross sectional orientation as in B, red box. D) Diagram of a lens equatorial cross-section. Epithelial cells (Epi) are on the periphery. Each hexagonal fiber cell (red asterisk) has 6 neighboring cells, colored orange, yellow or blue. There is a neighboring cell on each of the 4 short sides (1, 3, 4 and 6) and 2 broad sides (2 and 5). The orange cell (2) is a less mature cell (started elongating after the cell with the asterisk). The yellow cells (1, 3, 4 and 6) are about the same age, and the blue cell (5) is more mature (started elongating before the cell with asterisk). E–I) Phalloidin-stained cross-section showing hexagonal packing of cells as they mature. Red boxed regions in E indicate the approximate locations where (F–I) higher magnification images were obtained. F-actin surrounds the entire fiber cell membrane and is enriched at the vertices and short sides. In cortical fiber cells (F), F-actin staining appears discontinuous, but, as fiber cells mature (G–I), F-actin staining becomes smooth and continuous.
F-actin bundles are important for stabilizing fiber cells during elongation and migration (Fischer et al., 2000; Lee et al., 2000). At the posterior tips of differentiating secondary fibers, F-actin forms a dense mesh at the basal surface (Al-Ghoul et al., 2003; Weber and Menko, 2006) (Figure 1M), and N-cadherin/F-actin complexes are enriched at the vertices of hexagonal fiber cells, with myosin II and caldesmon, a contractile regulatory protein, located at the center connected to the N-cadherin/F-actin complexes on the lateral membranes (Bassnett et al., 1999). These F-actin bundles are aligned from one cell to the next, connected via N-cadherin cell-cell junctions, with paxillin, myosin light chain kinase (MLCK) and focal adhesion kinase also detected in these membrane complexes (Bassnett et al., 1999). Basal F-actin bundles at posterior fiber cell tips are thought to aid in the collective and coordinated migration of fiber cells along the lens capsule (Al-Ghoul et al., 2003; Bassnett et al., 1999; Weber and Menko, 2006). A recent study in zebrafish indicates fibronectin1 is localized in small puncta at the apical-apical junctions between epithelial cells and elongating tips of secondary lens fiber cells, as well as in posterior portions of secondary fiber cells (Hayes et al., 2012). Loss of fibronectin1 in zebrafish lenses leads to abnormal F-actin distribution, disorganization of secondary lens fibers and disrupted Y-sutures (Hayes et al., 2012), suggesting that integrin signaling via focal contacts leads to altered F-actin organization and impaired fiber cell migration.
As fiber cells differentiate, the discontinuous and irregular spectrin-actin network becomes smooth and continuous along fiber cell membranes (Figure 2E–2I), likely due to tropomodulin 1 (Tmod1) stabilization of F-actin after its assembly (Lee et al., 2000; Nowak et al., 2009; Nowak and Fowler, 2012). Tmod1, an actin pointed end capping protein first identified in human erythrocytes (Fowler, 1987; Moyer et al., 2010), is also required for maintaining the hexagonal geometry and packing of differentiating lens fiber cells in the mouse lens (Gokhin et al., 2012; Nowak and Fowler, 2012). The spectrin-actin network, also known as the membrane skeleton, and Tmod1 are associated in a macromolecular complex with CP49 and filensin, which form beaded intermediate filaments (Fischer et al., 2003b; Gokhin et al., 2012). Loss of either Tmod1 or CP49 causes decreased lens mechanical stiffness, presumably due to disruption of the spectrin-actin or beaded intermediate filament networks, respectively (Gokhin et al., 2012; Nowak et al., 2009). Unexpectedly, the membrane skeleton and beaded intermediate filaments synergize to promote the formation of large micron-size gap junction plaques in differentiating fiber cells that are crucial for normal lens ion and fluid homeostasis (Cheng et al., 2015). Gap junction plaques rest in lacunae within the spectrin-actin network along fiber cell membranes, suggesting that the membrane skeleton plays a role in accretion or stability of large gap junction plaques in the lens (Cheng et al., 2015). It remains unclear whether the membrane skeleton interacts directly with gap junction plaques, or if gap junction plaque stability/assembly is affected indirectly by membrane skeleton/beaded filament network interactions through a linker protein (Fuchs and Yang, 1999; Wiche et al., 2015; Wiche and Winter, 2011).
The entire spectrin-actin network is proposed to be tethered to the plasma membrane through spectrin interactions with ankyrin-B (Bennett and Baines, 2001; Bennett and Stenbuck, 1979, 1980; Luna and Hitt, 1992; More et al., 2001) as well as by ezrin-periplakin-periaxin-desmoyokin (EPPD)-actin anchorage complexes (Maddala et al., 2011b; Maddala et al., 2015b; Straub et al., 2003). Ankyrin-B links the membrane skeleton to NrCAM (More et al., 2001) and possibly N-cadherin, as shown for E-cadherin in other epithelial cell types (Kizhatil et al., 2007). The loss of NrCAM or ankyrin-B in the lens causes cataracts and disrupts the actin cytoskeleton (More et al., 2001), and a recent study shows that haploinsufficiency of ankyrin-B leads to a disrupted spectrin-actin network, abnormal lens fiber cell shape and decreased lens stiffness (Maddala et al., 2015b). Interestingly, ankyrin-B disruption in the lens also affects the levels of perixain. Similarly, in periaxin knockout lenses, there is a dramatic decrease in actin, spectrin and ankyrin-B protein levels and staining signals at the fiber cell membrane (Maddala et al., 2011b; Maddala et al., 2015b). In periaxin and ankyrin-B mutant lenses, packing and shape of fiber cells appears normal at 3 weeks of age, and dramatic defects in cell shape occur with age, indicating that EPPD complexes and ankyrin-B are not needed for the initial packing of fiber cells, but are required for maintaining fiber cell shape, perhaps by helping to stabilize the membrane skeleton (Maddala et al., 2015b).
Cadherin-based adherens junctions interact with the lens actin cytoskeleton to maintain epithelial cell morphology and polarity and to influence fiber cell differentiation (Leonard et al., 2011; Pontoriero et al., 2009). F-actin and adherens junctions exist in complexes between epithelial-epithelial, epithelial-fiber and fiber-fiber cell membrane contacts (Lo, 1988). Lens epithelial cells mainly express E-cadherin, while fiber cells utilize N-cadherin (Xu et al., 2002), and these cadherin junctions are associated with the actin cytoskeleton via the β-catenin linker (Cain et al., 2008; Leonard et al., 2011; Straub et al., 2003). N-cadherin becomes increasingly associated with cytoskeletal proteins during lens development (Leong et al., 2000), and the formation of N-cadherin junctions in epithelial cells undergoing differentiation into lens fiber cells triggers changes in the actin cytoskeleton (Ferreira-Cornwell et al., 2000). Dlg-1, a homolog of Drosophila tumor suppressor discs-large (dlg), colocalizes with N-cadherin and E-cadherin in mouse lens epithelial and fiber cells (Nguyen et al., 2005). Similar to lens N-cadherin conditional knockouts (Pontoriero et al., 2009), loss of Dlg-1, a PDZ (PSD-95-Dlg-ZO-1) domain protein, in lenses leads to vacuole formation and abnormal orientation of secondary fiber cells (Rivera et al., 2009). Dlg-1 knockout lens fibers display disrupted F-actin and N-cadherin staining along with decreased levels of α-catenin, a linker between cadherins and the actin cytoskeleton (Rivera et al., 2009). This study suggests that Dlg-1 is needed for normal adherens junctions and actin cytoskeleton organization in the lens.
Cortactin binds to and regulates assembly of F-actin by activating the actin nucleator Arp2/3 (Higgs and Pollard, 2001; Pollard et al., 2000; Ren et al., 2009; Uruno et al., 2001; Weaver et al., 2001). Both Arp3 and cortactin are recruited to N-cadherin junctions as fiber cells begin to undergo differentiation and are enriched at the vertices of these hexagonal cells. Arp3 only co-immunoprecipitates with N-cadherin and cortactin in lens fibers but not epithelial cells, suggesting that Arp3 plays a role in fiber cell differentiation to remodel cell-cell adhesions (Leonard et al., 2011). Experiments with an N-cadherin function-blocking antibody in embryonic chick lens explants shows that N-cadherin-directed F-actin assembly is required for fiber cell elongation (Leonard et al., 2011).
Rho GTPase family members regulate both cadherin junctions and F-actin assembly (Fukata et al., 1999; Nagafuchi et al., 1994). Activated Rac GTPase stimulates the c-abl-interactor (Abi) family of adaptor proteins that interact with c-abl kinase to regulate F-actin assembly and dynamics via WAVE-mediated Arp2/3 activation (Leng et al., 2005; Miki and Takenawa, 2003; Stradal et al., 2004; Stuart et al., 2006). Loss of either Rac1 or Abi-2 causes secondary fiber cell migration and orientation defects in neonatal lenses (Grove et al., 2004; Maddala et al., 2011a), demonstrating the importance of F-actin polymerization and organization during lens fiber cell migration and elongation. Abi may function as a link between adherens junctions and actin polymerization by interacting with both diaphanous (Dia)-related formins, which act as F-actin nucleators, and β-catenin, which regulate cadherin-based actin networks (Ryu et al., 2009). Interestingly, the overexpression of secreted frizzled-related protein 2 (Sfrp2), a Wnt signaling antagonist, also leads to secondary fiber cell orientation defects very similar to Rac1 or Abi-2 knockout lenses (Chen et al., 2008). There is a decrease in Rac1 staining in the cortex of transgenic Sfrp2 lenses along with a loss of RhoA and Cdc42 enrichment at fiber cell tips (Chen et al., 2008). Decreased protein levels of Rho GTPases in these Sfrp2 transgenic lenses provides evidence that Wnt/planar cell polarity pathways are important for F-actin cytoskeletal organization during fiber cell differentiation (Chen et al., 2008).
2.3 Mature secondary fiber cells
As secondary fiber cells undergo differentiation and maturation, domains of interlocking membrane protrusions with various conformations (balls-and-sockets, protrusions, paddles and square arrays) form between neighboring fiber cells along the broad and short sides (Dickson and Crock, 1972; Kistler et al., 1986; Kuszak et al., 1980; Kuwabara, 1975; Lo and Harding, 1984; Willekens and Vrensen, 1981, 1982, 1985). Along broad sides of cortical differentiating fiber cells, ball-and-socket membrane interdigitations consist of a small protrusion on one cell precisely fitted into the socket indentation of the neighboring cell, and these processes increase in size as fiber cells mature (Harding et al., 1976; Kuszak et al., 1980; Lo and Reese, 1993; Willekens and Vrensen, 1981, 1982, 1985). In addition to balls-and-sockets along broad sides, cortical fiber cells also develop small interlocking protrusions at their vertices (tricellular junctions) adjacent to the short sides (Kistler et al., 1986; Kuszak et al., 1980; Kuwabara, 1975; Leeson, 1971; Lo et al., 2014; Zhou and Lo, 2003). Actin is highly enriched in small interlocking protrusions at these vertices (Lo et al., 1997; Zhou and Lo, 2003), but its role has not been well studied in balls-and-socket protrusions on the broad sides.
Early stages in small protrusion formation at the vertices are believed to involve plasma membrane invagination similar to clathrin-AP2-mediated endocytic pit formation (Kirchhausen, 1999, 2000; Kirchhausen et al., 1986; Pearse et al., 2000; Smith and Pearse, 1999). Indeed, clathrin and AP2 coat the cytoplasmic surface of fiber cell membrane indentations, and F-actin is observed on the cytoplasmic surface of the neighboring fiber cell membrane inside each protrusion (Zhou and Lo, 2003). It has been hypothesized that Arp2/3 may drive branching of F-actin in protrusions to generate a pushing force that would be coordinated with the pulling force generated by clathrin complexes along the concave membrane surface enveloping protrusions from the adjacent cell. The branched F-actin network would then presumably stabilize the interlocking protrusion/invagination structure (Zhou and Lo, 2003). Loss of Tmod1 and CP49 leads to a reduction in small protrusions at the vertices of mature fiber cells (Nowak et al., 2009), suggesting that both actin and intermediate filaments are needed for normal formation or stabilization of these interdigitations. Recent studies have localized aquaporin-0 and N-cadherin to small protrusions in mature fiber cells (Biswas et al., 2015; Lo et al., 2014), suggesting that these membrane proteins may be required for normal formation of protrusions at fiber cell vertices. However, the pathways and mechanisms that regulate the formation of these complex fiber cell interdigitations remain unclear.
As fiber cells continue to mature, they undergo complex degradative processes to eliminate all intracellular organelles, including nuclei, to remove light-scattering structures from the light path (Bassnett, 1992, 1995; Bassnett and Beebe, 1992; Bassnett and Mataic, 1997). Along with the removal of cellular organelles, there is degradation and/or proteolytic trimming of many cytoskeletal proteins, including microtubules, vimentin intermediate filaments and beaded intermediate filaments (Blankenship et al., 2001; Bradley et al., 1979; FitzGerald, 2009; Kuwabara, 1968; Oka et al., 2008; Rafferty, 1985; Sandilands et al., 1995). While α2- and β2-spectrin are cleaved by calpains and caspases into two large fragments (De Maria et al., 2009; Lee et al., 2000; Lee et al., 2001; Nowak et al., 2009), F-actin and Tmods (Tmod1 in mouse lenses and Tmod4 in chicken lenses) are not proteolysed and remain associated with the plasma membranes of mature fiber cells after denucleation and organelle loss (Lee et al., 2000; Lee et al., 2001; Nowak et al., 2009). These data suggest that the F-actin network likely plays a role in stabilizing and maintaining membrane integrity of mature fiber cells.
Maintenance of the actin cytoskeleton in mature fiber cells may rely on appropriate interactions with lens crystallin proteins. Crystallins comprise 90% of total lens proteins and are classified as α-, β- and γ-crystallins (Bloemendal et al., 2004). Alpha-crystallins, consisting of heteromers with αA- and αB-crystallin subunits, belong to the small heat shock protein family and are hypothesized to help maintain life-long lens transparency by sequestering denatured proteins and thereby preventing abnormal protein aggregation (Horwitz, 1992). Alpha-crystallins are reported to be associated with actin in lens lysates (Bloemendal et al., 1984; Del Vecchio et al., 1984; Gopalakrishnan and Takemoto, 1992; Kibbelaar et al., 1979), and mutations in αA-crystallin alter its association with actin (Andley et al., 2014; Brown et al., 2007), suggesting that actin may be a native substrate of α-crystallins. Beta- and γ-crystallins are members of the β/γ superfamily of proteins and function as structural proteins in the lens (Bloemendal et al., 2004). Immunoprecipitation experiments reveal a possible link between β- and γ-crystallins and major cytoskeletal proteins in the lens, including actin (Rao et al., 2008). Gamma-crystallins may play a role in stabilizing F-actin in mature lens fiber cells. In mature fiber cells, the γB-S11R mutation leads to reduced F-actin staining (Li et al., 2008) while loss of γS-crystallin in mouse lenses leads to F-actin depletion and aggregation (Fan et al., 2012). In vitro experiments suggest that γS-crystallins stabilize F-actin and protect filaments against depolymerization (Fan et al., 2012).
3. Approaches and future directions for studying actin in lens cells
3.1 Actin cytoskeleton networks and actin-associated proteins in the lens
While it is clear that actin plays important and diverse roles during lens development and in maintaining lens integrity, there remain a host of unknowns about actin organization in the lens. In other systems, such as striated muscle, precise locations of actin filament ends and associated cross-linking and binding proteins have been documented in detail (Clark et al., 2002; Ono, 2010). We can use similar techniques to reveal the structural organization of the actin cytoskeleton in the lens. Comparing the localization of barbed-end capping proteins, such as adducin (Kaiser et al., 1989; Matsuoka et al., 2000), CapZ (dos Remedios et al., 2003) or gelsolin (Andley et al., 2014; Nag et al., 2013), vs. the pointed-end capping protein Tmod1 (Nowak et al., 2009; Woo and Fowler, 1994) could help establish locations of barbed and pointed ends of actin filaments, respectively, although this may require super-resolution microscopy approaches if filaments are short and/or arranged in irregular orientations (see below).
The locations of actin filament side-binding proteins can also shed light on the organization of actin cytoskeletal networks. Alpha-actinin is a crosslinking protein for anti-parallel actin filaments that interact with myosin II bipolar filaments in loose bundles to generate contractile force (FitzGerald and Casselman, 1990; Lo et al., 1997; Sjoblom et al., 2008). F-actin bundles containing α-actinin are found in cortical lens fiber cells and are commonly localized near the vertices on the short sides of hexagonal fiber cells, perhaps serving to stabilize the hexagonal cell shape (Lo et al., 1997). The functions of these bundles, or whether other actin-associated proteins are associated with bundled or non-bundled F-actin in fiber cells, remain to be studied. For example, the presence of other actin-bundling proteins, such fascin (Jayo and Parsons, 2010) and fimbrin (aka plastin) (Delanote et al., 2005), would indicate bundles of filaments aligned in parallel orientations, while the presence of filamin (Hirata et al., 2014), would indicate looser networks of isotropically oriented filaments, each with distinct structural, mechanical and cellular functions (Matsudaira, 1994; Ridley, 2011; Tseng et al., 2005).
Locations of actin-nucleating proteins, such as Arp2/3 or formins, or depolymerizing proteins, such as ADF/cofilin, could indicate F-actin networks undergoing active assembly/disassembly and remodeling in specific cells or subcellular regions (Carlier et al., 2015; Ono, 2007; Pollard, 2007; Pollard and Cooper, 2009). Current data suggest that dynamic branched F-actin networks nucleated by Arp2/3, and α-actinin-stabilized anti-parallel actin filament bundles are both present at or near the vertices of fiber cells (Leonard et al., 2011; Lo et al., 1997), implying the complexity of F-actin organization and function at these fiber cell domains. While formins have been detected in developing lens tissue, and loss of formins may lead to fiber cell degeneration (de la Pompa et al., 1995; Woychik et al., 1990), it remains unclear which isoforms of this large and diverse family are required for establishing and maintaining the lens.
Another important and relatively poorly understood area is how F-actin networks are linked to plasma membranes of lens cells to control their complex topologies and functional domains. Ezrin-radixin-moesin (ERM) proteins, which link actin filament networks to the plasma membrane (Bretscher et al., 2000) and interact with N-WASP, an activator of Arp2/3-nucleated actin assembly (Manchanda et al., 2005), are present in the lens (Bagchi et al., 2004; Ingraffea et al., 2002; Rao et al., 2008; Straub et al., 2003). Immunoprecipitation experiments (Straub et al., 2003) indicate that plectin may serve as an important linker between ERM proteins, intermediate filament (Wiche et al., 2015; Wiche and Winter, 2011) and actin filament networks (Andra et al., 1998; Fontao et al., 2001; Jiu et al., 2015) in the lens. A recent study indicates that polymorphisms and genetic variations in ezrin are linked to human age-related cataracts (Lin et al., 2013), but the mechanism for these opacities and any links to the actin cytoskeleton will require further study. In addition, more work is needed to understand the subcellular localization and functions of other components of the spectrin-based membrane skeleton in the lens, such as tropomyosin (Nowak et al., 2009; Woo et al., 2000), band 3 (Allen et al., 1987), band 4.1 (Aster et al., 1986; Aster et al., 1984; Bagchi et al., 2004; Beebe et al., 2001), band 4.2 (Sung and Lo, 1997) and band 4.9 (Faquin et al., 1988).
Super-resolution microscopy techniques, such as structured illumination microscopy (SIM) (Brown et al., 2011; Gao et al., 2012; Gustafsson, 2000) and stimulated emission depletion (STED) microscopy (Chereau et al., 2015; Hell and Wichmann, 1994), could reveal more precise localization of F-actin and actin-binding proteins to determine whether multiple types of actin networks overlap at fiber cell vertices and/or along cell broad and narrow sides, or form distinct subdomains. In addition, recent advances in correlative light and electron microscopy (CLEM) can couple information about protein localization obtained using fluorescence microscopy with highly detailed ultrastructural electron microscopy information (de Boer et al., 2015) to revolutionize the study of F-actin and other proteins required to form the complex membrane geometry of fiber cell interdigitations, and to coordinate diverse F-actin structures at the interfaces between migrating fiber cell tips and the apical domains of lens epithelial cells.
3.2 F-actin dynamics and stability
In most cells (Pollard et al., 2000; Pollard and Borisy, 2003), including lens cells, a large fraction of actin remains unassembled and associated with monomer-sequestering proteins, but is available for filament assembly upon appropriate signals, to coordinate cell migration, adhesion and other morphogenesis events (Ireland et al., 1983; Ramaekers et al., 1981). While previous studies have demonstrated that G- to F-actin assembly driven by signaling through the Rho family of proteins (Sections 1 and 2.2) is needed for lens development and fiber cell elongation, it remains unclear whether the ratio of G-actin to F-actin is important to maintain adult lens homeostasis and integrity. By comparing relative proportions of cytosolic G-actin vs. membrane-bound F-actin in subcellular fractions of lens lysates (Nowak et al., 2009; Woo et al., 2000), or evaluating the G/F-actin ratio in lysates using DNaseI (Blikstad et al., 1978; Kibbelaar et al., 1979; Ramaekers et al., 1981), we can investigate whether genetic mutations or lens aging affects global actin polymerization or filament stability. In addition, comparison of relative fluorescence intensity levels of phalloidin vs. DNase I staining can be used to reveal the F- to G-actin ratio (i.e., relative extent of F-actin polymerization) (Fischer et al., 2003a) in specific layers of lens fiber cells (Fan et al., 2012). Here we note that actin has often been used as a loading control for Western blots and as a membrane marker for lens sections. However, as we have illustrated in this review, many proteins directly interact with or indirectly affect the actin cytoskeleton. Therefore, using actin as a control is likely not appropriate for all studies. We suggest the use of Ponceau S staining for total proteins as a loading control for Western blots (Gilda and Gomes, 2013), while fluorescently labeled wheat germ agglutinin is a bright and easy-to-use membrane marker that outlines lens epithelial and fiber cells in fluorescent microscope images (Bond et al., 1996).
Another powerful approach to elucidate F-actin network assembly, stability and functions in the lens is the use of actin- or myosin-disrupting drugs. For example, disruption of myosin II binding to F-actin via blebbistatin inhibition of myosin ATPase (Kovacs et al., 2004), or via ML-7 inhibition of MLCK activity, leads to a decrease in whole lens stiffness (Won et al., 2015) and changes in focal length in chick lenses (Luck and Choh, 2011), and causes nuclear cataracts in mouse lenses due to changes in fiber cell morphology (Maddala et al., 2007). Treatment of chick lenses with latrunculin A, which binds and sequesters G-actin, preventing actin polymerization (Coue et al., 1987; Morton et al., 2000; Spector et al., 1989), also leads to a decrease in lens stiffness (Won et al., 2015), suggesting that a dynamic F-actin network is important for maintaining lens mechanical integrity. In addition to actin- and myosin-disrupting agents, there are drugs that stabilize F-actin, such as jaspaklinolide (Allingham et al., 2006; Bubb et al., 1994; Bubb et al., 2000; Holzinger, 2009), or specifically inhibit Arp2/3, formins or Rho GTPases, that will provide useful information about the functions of lens actin networks (Blanchoin and Boujemaa-Paterski, 2009; Hetrick et al., 2013; Nolen et al., 2009; Rizvi et al., 2009; Shang et al., 2013; Shang et al., 2012). While treatment of whole lenses can indicate global functions for F-actin assembly pathways and structural networks, information about F-actin in the lens with respect to regional cellular specializations and functions will require careful microscopic analyses of epithelial and fiber cells after drug treatments.
The availability of transgenic mice with GFP-tagged cytoskeletal proteins offers yet another approach to studying actin dynamics regulation in the lens. By coupling high-resolution confocal or multi-photon fluorescence microscopy and transgenic mice expressing GFP-tagged actin (Gurniak and Witke, 2007; Narayanan et al., 2015), or GFP- or mRFPruby-tagged Lifeact, a F-actin binding peptide (Riedl et al., 2010), or other fluorescently-tagged F-actin-binding proteins (e.g., moesin or paxillin) (Abe et al., 2011), it would be possible to study how the actin cytoskeleton is remodeled during the epithelial-to-fiber-cell transition and during fiber cell elongation, migration and maturation in live lenses. Recent advances in the CRISPR-Cas9 technology (Sternberg and Doudna, 2015) will also greatly increase the number of knockout, transgenic and mutant animal models that can be applied to the study of the lens actin cytoskeleton.
Concluding remarks
While it is clear that F-actin plays important roles during lens development, many questions remain about the mechanisms that maintain actin cytoskeletal structures in quiescent anterior epithelial cells and those that drive F-actin remodeling as equatorial epithelial cells proliferate, differentiate and mature into fiber cells. Many components of F-actin networks have been detected in the lens, but the functions of most of these proteins in establishing or maintaining lens cellular architecture and functions remain unknown. Further studies are also needed to identify additional proteins that directly interact with the actin cytoskeleton to regulate dynamic G-to-F-actin transitions or network rearrangements during lens fiber cell differentiation, and during lens development and aging. Fiber cells at the center of the lens are formed during embryogenesis, and these long-lived cells must maintain homeostasis and transparency throughout the lifetime of an organism. Little is known about the role of the actin cytoskeleton in maintaining the life-long integrity of mature lens fiber cells, and whether changes in the actin cytoskeleton or the membrane skeleton affect age-related pathologies, including cataracts and presbyopia. New methods, including super-resolution and live-cell microscopy, transgenic and knockout mouse models and novel actin-disrupting agents, present opportunities to study and understand actin cytoskeletal regulation and dynamics in live lenses.
Highlights.
-
-
F-actin plays important roles during lens development and maintains fiber cells.
-
-
Dynamic regulation of actin is needed for normal development and fiber elongation.
-
-
Actin filament stability and reorganization are essential for fiber cell packing.
-
-
Disruptions of actomyosin function affect lens biomechanical properties.
Acknowledgments
This work was supported by National Eye Institute grant R01 EY017724 (VMF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Kiyonari H, Shioi G, Inoue K, Nakao K, Aizawa S, Fujimori T. Establishment of conditional reporter mouse lines at ROSA26 locus for live cell imaging. Genesis. 2011;49:579–590. doi: 10.1002/dvg.20753. [DOI] [PubMed] [Google Scholar]
- Al-Ghoul KJ, Kuszak JR, Lu JY, Owens MJ. Morphology and organization of posterior fiber ends during migration. Molecular vision. 2003;9:119–128. [PubMed] [Google Scholar]
- Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG. Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin. Investigative ophthalmology & visual science. 2003;44:5252–5258. doi: 10.1167/iovs.03-0224. [DOI] [PubMed] [Google Scholar]
- Allen DP, Low PS, Dola A, Maisel H. Band 3 and ankyrin homologues are present in eye lens: evidence for all major erythrocyte membrane components in same non-erythroid cell. Biochemical and biophysical research communications. 1987;149:266–275. doi: 10.1016/0006-291x(87)91634-2. [DOI] [PubMed] [Google Scholar]
- Allingham JS, Klenchin VA, Rayment I. Actin-targeting natural products: structures, properties and mechanisms of action. Cellular and molecular life sciences : CMLS. 2006;63:2119–2134. doi: 10.1007/s00018-006-6157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almenar-Queralt A, Lee A, Conley CA, Ribas de Pouplana L, Fowler VM. Identification of a novel tropomodulin isoform, skeletal tropomodulin, that caps actin filament pointed ends in fast skeletal muscle. The Journal of biological chemistry. 1999;274:28466–28475. doi: 10.1074/jbc.274.40.28466. [DOI] [PubMed] [Google Scholar]
- Amann KJ, Pollard TD. Direct real-time observation of actin filament branching mediated by Arp2/3 complex using total internal reflection fluorescence microscopy. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:15009–15013. doi: 10.1073/pnas.211556398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andley UP, Malone JP, Townsend RR. In vivo substrates of the lens molecular chaperones alphaA-crystallin and alphaB-crystallin. PloS one. 2014;9:e95507. doi: 10.1371/journal.pone.0095507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andra K, Nikolic B, Stocher M, Drenckhahn D, Wiche G. Not just scaffolding: plectin regulates actin dynamics in cultured cells. Genes & development. 1998;12:3442–3451. doi: 10.1101/gad.12.21.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aster JC, Brewer GJ, Maisel H. The 4.1-like proteins of the bovine lens: spectrin-binding proteins closely related in structure to red blood cell protein 4.1. The Journal of cell biology. 1986;103:115–122. doi: 10.1083/jcb.103.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aster JC, Welsh MJ, Brewer GJ, Maisel H. Identification of spectrin and protein 4.1-like proteins in mammalian lens. Biochemical and biophysical research communications. 1984;119:726–734. doi: 10.1016/s0006-291x(84)80311-3. [DOI] [PubMed] [Google Scholar]
- Bagchi M, Katar M, Lo WK, Yost R, Hill C, Maisel H. ERM proteins of the lens. Journal of cellular biochemistry. 2004;92:626–630. doi: 10.1002/jcb.20062. [DOI] [PubMed] [Google Scholar]
- Bamburg JR, Harris HE, Weeds AG. Partial purification and characterization of an actin depolymerizing factor from brain. FEBS letters. 1980;121:178–182. doi: 10.1016/0014-5793(80)81292-0. [DOI] [PubMed] [Google Scholar]
- Bassnett S. Mitochondrial dynamics in differentiating fiber cells of the mammalian lens. Current eye research. 1992;11:1227–1232. doi: 10.3109/02713689208999548. [DOI] [PubMed] [Google Scholar]
- Bassnett S. The fate of the Golgi apparatus and the endoplasmic reticulum during lens fiber cell differentiation. Investigative ophthalmology & visual science. 1995;36:1793–1803. [PubMed] [Google Scholar]
- Bassnett S. Three-dimensional reconstruction of cells in the living lens: the relationship between cell length and volume. Experimental eye research. 2005;81:716–723. doi: 10.1016/j.exer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Beebe DC. Coincident loss of mitochondria and nuclei during lens fiber cell differentiation. Developmental dynamics : an official publication of the American Association of Anatomists. 1992;194:85–93. doi: 10.1002/aja.1001940202. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Mataic D. Chromatin degradation in differentiating fiber cells of the eye lens. The Journal of cell biology. 1997;137:37–49. doi: 10.1083/jcb.137.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, Missey H, Vucemilo I. Molecular architecture of the lens fiber cell basal membrane complex. Journal of cell science. 1999;112(Pt 13):2155–2165. doi: 10.1242/jcs.112.13.2155. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Winzenburger PA. Morphometric analysis of fibre cell growth in the developing chicken lens. Experimental eye research. 2003;76:291–302. doi: 10.1016/s0014-4835(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Cerrelli S. Cytochalasin prevents cell elongation and increases potassium efflux from embryonic lens epithelial cells: implications for the mechanism of lens fiber cell elongation. Lens and eye toxicity research. 1989;6:589–601. [PubMed] [Google Scholar]
- Beebe DC, Vasiliev O, Guo J, Shui YB, Bassnett S. Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Investigative ophthalmology & visual science. 2001;42:727–734. [PubMed] [Google Scholar]
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiological reviews. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- Bennett V, Stenbuck PJ. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. The Journal of biological chemistry. 1979;254:2533–2541. [PubMed] [Google Scholar]
- Bennett V, Stenbuck PJ. Human erythrocyte ankyrin. Purification and properties. The Journal of biological chemistry. 1980;255:2540–2548. [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. The Biochemical journal. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Son A, Yu Q, Zhou R, Lo WK. Breakdown of interlocking domains may contribute to formation of membranous globules and lens opacity in ephrin-A5 mice. Experimental eye research. 2015;145:130–139. doi: 10.1016/j.exer.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L, Amann KJ, Higgs HN, Marchand JB, Kaiser DA, Pollard TD. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 2000;404:1007–1011. doi: 10.1038/35010008. [DOI] [PubMed] [Google Scholar]
- Blanchoin L, Boujemaa-Paterski R. Inhibitors target actin nucleators. Chemistry & biology. 2009;16:1125–1126. doi: 10.1016/j.chembiol.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Blankenship TN, Hess JF, FitzGerald PG. Development- and differentiation-dependent reorganization of intermediate filaments in fiber cells. Investigative ophthalmology & visual science. 2001;42:735–742. [PubMed] [Google Scholar]
- Blikstad I, Markey F, Carlsson L, Persson T, Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978;15:935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, Berbers GA, De Jong WW, Ramaekers FC, Vermorken AJ, Dunia I, Benedetti EL. Interaction of crystallins with the cytoskeletal-plasma membrane complex of the bovine lens. Ciba Foundation symposium. 1984;106:177–190. doi: 10.1002/9780470720875.ch10. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Progress in biophysics and molecular biology. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Bond J, Green C, Donaldson P, Kistler J. Liquefaction of cortical tissue in diabetic and galactosemic rat lenses defined by confocal laser scanning microscopy. Investigative ophthalmology & visual science. 1996;37:1557–1565. [PubMed] [Google Scholar]
- Borges RM, Lamers ML, Forti FL, Santos MF, Yan CY. Rho signaling pathway and apical constriction in the early lens placode. Genesis. 2011;49:368–379. doi: 10.1002/dvg.20723. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Ireland M, Maisel H. The cytoskeleton of chick lens cells. Experimental eye research. 1979;28:441–453. doi: 10.1016/0014-4835(79)90119-2. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. The Journal of cell biology. 1983;97:425–432. doi: 10.1083/jcb.97.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Nguyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annual review of cell and developmental biology. 2000;16:113–143. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- Brown AC, Oddos S, Dobbie IM, Alakoskela JM, Parton RM, Eissmann P, Neil MA, Dunsby C, French PM, Davis I, Davis DM. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS biology. 2011;9:e1001152. doi: 10.1371/journal.pbio.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Z, Ponce A, Lampi K, Hancock L, Takemoto L. Differential binding of mutant (R116C) and wildtype alphaA crystallin to actin. Current eye research. 2007;32:1051–1054. doi: 10.1080/02713680701769989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. The Journal of biological chemistry. 1994;269:14869–14871. [PubMed] [Google Scholar]
- Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. The Journal of biological chemistry. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- Cain S, Martinez G, Kokkinos MI, Turner K, Richardson RJ, Abud HE, Huelsken J, Robinson ML, de Iongh RU. Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Developmental biology. 2008;321:420–433. doi: 10.1016/j.ydbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Carlier MF, Pernier J, Montaville P, Shekhar S, Kuhn S, Cytoskeleton D, Motility g. Control of polarized assembly of actin filaments in cell motility. Cellular and molecular life sciences : CMLS. 2015;72:3051–3067. doi: 10.1007/s00018-015-1914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BK, Disanza A, Choi SY, Faber SC, Lou M, Beggs HE, Scita G, Zheng Y, Lang RA. Cdc42- and IRSp53-dependent contractile filopodia tether presumptive lens and retina to coordinate epithelial invagination. Development. 2009;136:3657–3667. doi: 10.1242/dev.042242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, McAvoy JW. A role for Wnt/planar cell polarity signaling during lens fiber cell differentiation? Seminars in cell & developmental biology. 2006;17:712–725. doi: 10.1016/j.semcdb.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, Shimono A, McAvoy JW. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Developmental biology. 2008;324:161–176. doi: 10.1016/j.ydbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Ansari MM, Cooper JA, Gong X. EphA2 and Src regulate equatorial cell morphogenesis during lens development. Development. 2013;140:4237–4245. doi: 10.1242/dev.100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Nowak RB, Gao J, Sun X, Biswas SK, Lo WK, Mathias RT, Fowler VM. Lens ion homeostasis relies on the assembly and/or stability of large connexin 46 gap junction plaques on the broad sides of differentiating fiber cells. American journal of physiology. Cell physiology. 2015;308:C835–C847. doi: 10.1152/ajpcell.00372.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereau R, Tonnesen J, Nagerl UV. STED microscopy for nanoscale imaging in living brain slices. Methods. 2015 doi: 10.1016/j.ymeth.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annual review of cell and developmental biology. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS letters. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- de Boer P, Hoogenboom JP, Giepmans BN. Correlated light and electron microscopy: ultrastructure lights up! Nature methods. 2015;12:503–513. doi: 10.1038/nmeth.3400. [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, James D, Zeller R. Limb deformity proteins during avian neurulation and sense organ development. Developmental dynamics : an official publication of the American Association of Anatomists. 1995;204:156–167. doi: 10.1002/aja.1002040206. [DOI] [PubMed] [Google Scholar]
- De Maria A, Shi Y, Kumar NM, Bassnett S. Calpain expression and activity during lens fiber cell differentiation. The Journal of biological chemistry. 2009;284:13542–13550. doi: 10.1074/jbc.M900561200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rocco D, Zieger B, Platokouki H, Heller PG, Pastore A, Bottega R, Noris P, Barozzi S, Glembotsky AC, Pergantou H, Balduini CL, Savoia A, Pecci A. MYH9-related disease: five novel mutations expanding the spectrum of causative mutations and confirming genotype/phenotype correlations. European journal of medical genetics. 2013;56:7–12. doi: 10.1016/j.ejmg.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio PJ, MacElroy KS, Rosser MP, Church RL. Association of alpha-crystallin with actin in cultured lens cells. Current eye research. 1984;3:1213–1219. doi: 10.3109/02713688409000824. [DOI] [PubMed] [Google Scholar]
- Delanote V, Vandekerckhove J, Gettemans J. Plastins: versatile modulators of actin organization in (patho)physiological cellular processes. Acta pharmacologica Sinica. 2005;26:769–779. doi: 10.1111/j.1745-7254.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- Dickson DH, Crock GW. Interlocking patterns on primate lens fibers. Investigative ophthalmology. 1972;11:809–815. [PubMed] [Google Scholar]
- dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiological reviews. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Economou M, Batzios SP, Pecci A, Printza N, Savoia A, Barozzi S, Theodoridou S, Teli A, Psillas G, Zafeiriou DI. MYH9-related disorders: report on a patient of Greek origin presenting with macroscopic hematuria and presenile cataract, caused by an R1165C mutation. Journal of pediatric hematology/oncology. 2012;34:412–415. doi: 10.1097/MPH.0b013e318257a64b. [DOI] [PubMed] [Google Scholar]
- Ehrlich JS, Hansen MD, Nelson WJ. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Developmental cell. 2002;3:259–270. doi: 10.1016/s1534-5807(02)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Fan J, Dong L, Mishra S, Chen Y, FitzGerald P, Wistow G. A role for gammaS-crystallin in the organization of actin and fiber cell maturation in the mouse lens. The FEBS journal. 2012;279:2892–2904. doi: 10.1111/j.1742-4658.2012.08669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faquin WC, Husain A, Hung J, Branton D. An immunoreactive form of erythrocyte protein 4.9 is present in non-erythroid cells. European journal of cell biology. 1988;46:168–175. [PubMed] [Google Scholar]
- Ferreira-Cornwell MC, Veneziale RW, Grunwald GB, Menko AS. N-cadherin function is required for differentiation-dependent cytoskeletal reorganization in lens cells in vitro. Experimental cell research. 2000;256:237–247. doi: 10.1006/excr.2000.4819. [DOI] [PubMed] [Google Scholar]
- Fischer RS, Fritz-Six KL, Fowler VM. Pointed-end capping by tropomodulin3 negatively regulates endothelial cell motility. The Journal of cell biology. 2003a;161:371–380. doi: 10.1083/jcb.200209057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RS, Lee A, Fowler VM. Tropomodulin and tropomyosin mediate lens cell actin cytoskeleton reorganization in vitro. Investigative ophthalmology & visual science. 2000;41:166–174. [PubMed] [Google Scholar]
- Fischer RS, Quinlan RA, Fowler VM. Tropomodulin binds to filensin intermediate filaments. FEBS letters. 2003b;547:228–232. doi: 10.1016/s0014-5793(03)00711-7. [DOI] [PubMed] [Google Scholar]
- FitzGerald PG. Lens intermediate filaments. Experimental eye research. 2009;88:165–172. doi: 10.1016/j.exer.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald PG, Casselman J. Discrimination between the lens fiber cell 115 kd cytoskeletal protein and alpha-actinin. Current eye research. 1990;9:873–882. doi: 10.3109/02713689008999559. [DOI] [PubMed] [Google Scholar]
- Fontao L, Geerts D, Kuikman I, Koster J, Kramer D, Sonnenberg A. The interaction of plectin with actin: evidence for cross-linking of actin filaments by dimerization of the actin-binding domain of plectin. Journal of cell science. 2001;114:2065–2076. doi: 10.1242/jcs.114.11.2065. [DOI] [PubMed] [Google Scholar]
- Fowler VM. Identification and purification of a novel Mr 43,000 tropomyosin-binding protein from human erythrocyte membranes. The Journal of biological chemistry. 1987;262:12792–12800. [PubMed] [Google Scholar]
- Fowler VM. Regulation of actin filament length in erythrocytes and striated muscle. Current opinion in cell biology. 1996;8:86–96. doi: 10.1016/s0955-0674(96)80052-4. [DOI] [PubMed] [Google Scholar]
- Fowler VM. The human erythrocyte plasma membrane: a Rosetta Stone for decoding membrane-cytoskeleton structure. Current topics in membranes. 2013;72:39–88. doi: 10.1016/B978-0-12-417027-8.00002-7. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Yang Y. Crossroads on cytoskeletal highways. Cell. 1999;98:547–550. doi: 10.1016/s0092-8674(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Fudge DS, McCuaig JV, Van Stralen S, Hess JF, Wang H, Mathias RT, FitzGerald PG. Intermediate filaments regulate tissue size and stiffness in the murine lens. Investigative ophthalmology & visual science. 2011;52:3860–3867. doi: 10.1167/iovs.10-6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsuura Y, Yonehara S, Fujisawa H, Kikuchi A, Kaibuchi K. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. The Journal of biological chemistry. 1999;274:26044–26050. doi: 10.1074/jbc.274.37.26044. [DOI] [PubMed] [Google Scholar]
- Gao L, Shao L, Higgins CD, Poulton JS, Peifer M, Davidson MW, Wu X, Goldstein B, Betzig E. Noninvasive imaging beyond the diffraction limit of 3D dynamics in thickly fluorescent specimens. Cell. 2012;151:1370–1385. doi: 10.1016/j.cell.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilda JE, Gomes AV. Stain-Free total protein staining is a superior loading control to beta-actin for Western blots. Analytical biochemistry. 2013;440:186–188. doi: 10.1016/j.ab.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin DS, Nowak RB, Kim NE, Arnett EE, Chen AC, Sah RL, Clark JI, Fowler VM. Tmod1 and CP49 synergize to control the fiber cell geometry, transparency, and mechanical stiffness of the mouse lens. PloS one. 2012;7:e48734. doi: 10.1371/journal.pone.0048734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annual review of biochemistry. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S, Takemoto L. Binding of actin to lens alpha crystallins. Current eye research. 1992;11:929–933. doi: 10.3109/02713689209033490. [DOI] [PubMed] [Google Scholar]
- Grove M, Demyanenko G, Echarri A, Zipfel PA, Quiroz ME, Rodriguiz RM, Playford M, Martensen SA, Robinson MR, Wetsel WC, Maness PF, Pendergast AM. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Molecular and cellular biology. 2004;24:10905–10922. doi: 10.1128/MCB.24.24.10905-10922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurniak CB, Witke W. HuGE, a novel GFP-actin-expressing mouse line for studying cytoskeletal dynamics. European journal of cell biology. 2007;86:3–12. doi: 10.1016/j.ejcb.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Gustafsson MG. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. Journal of microscopy. 2000;198:82–87. doi: 10.1046/j.1365-2818.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- Hao J, Kunishima S, Guo X, Hu R, Gao W. A large family with MYH9 disorder caused by E1841K mutation suffering from serious kidney and hearing impairment and cataracts. Annals of hematology. 2012;91:1147–1148. doi: 10.1007/s00277-011-1370-5. [DOI] [PubMed] [Google Scholar]
- Harding CV, Susan S, Murphy H. Scanning Electron Microscopy of the Adult Rabbit Lens. Ophthalmic research. 1976;8:443–455. [Google Scholar]
- Hayes JM, Hartsock A, Clark BS, Napier HR, Link BA, Gross JM. Integrin alpha5/fibronectin1 and focal adhesion kinase are required for lens fiber morphogenesis in zebrafish. Molecular biology of the cell. 2012;23:4725–4738. doi: 10.1091/mbc.E12-09-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nature reviews. Molecular cell biology. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Optics letters. 1994;19:780–782. doi: 10.1364/ol.19.000780. [DOI] [PubMed] [Google Scholar]
- Helwani FM, Kovacs EM, Paterson AD, Verma S, Ali RG, Fanning AS, Weed SA, Yap AS. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. The Journal of cell biology. 2004;164:899–910. doi: 10.1083/jcb.200309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetrick B, Han MS, Helgeson LA, Nolen BJ. Small molecules CK-666 and CK-869 inhibit actin-related protein 2/3 complex by blocking an activating conformational change. Chemistry & biology. 2013;20:701–712. doi: 10.1016/j.chembiol.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs HN, Pollard TD. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annual review of biochemistry. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- Hirata H, Sokabe M, Lim CT. Molecular mechanisms underlying the force-dependent regulation of actin-to-ECM linkage at the focal adhesions. Progress in molecular biology and translational science. 2014;126:135–154. doi: 10.1016/B978-0-12-394624-9.00006-3. [DOI] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Molecular and cellular biology. 2004;24:6690–6700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A. Jasplakinolide: an actin-specific reagent that promotes actin polymerization. Methods in molecular biology. 2009;586:71–87. doi: 10.1007/978-1-60761-376-3_4. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraffea J, Reczek D, Bretscher A. Distinct cell type-specific expression of scaffolding proteins EBP50 and E3KARP: EBP50 is generally expressed with ezrin in specific epithelia, whereas E3KARP is not. European journal of cell biology. 2002;81:61–68. doi: 10.1078/0171-9335-00218. [DOI] [PubMed] [Google Scholar]
- Ireland M, Lieska N, Maisel H. Lens actin: purification and localization. Experimental eye research. 1983;37:393–408. doi: 10.1016/0014-4835(83)90176-8. [DOI] [PubMed] [Google Scholar]
- Jayo A, Parsons M. Fascin: a key regulator of cytoskeletal dynamics. The international journal of biochemistry & cell biology. 2010;42:1614–1617. doi: 10.1016/j.biocel.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Jiu Y, Lehtimaki J, Tojkander S, Cheng F, Jaalinoja H, Liu X, Varjosalo M, Eriksson JE, Lappalainen P. Bidirectional Interplay between Vimentin Intermediate Filaments and Contractile Actin Stress Fibers. Cell reports. 2015;11:1511–1518. doi: 10.1016/j.celrep.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Jullien-Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis JH. Bridging Ral GTPase to Rho pathways. RLIP76, a Ral effector with CDC42/Rac GTPase-activating protein activity. The Journal of biological chemistry. 1995;270:22473–22477. doi: 10.1074/jbc.270.38.22473. [DOI] [PubMed] [Google Scholar]
- Kaiser HW, O'Keefe E, Bennett V. Adducin: Cadependent association with sites of cell-cell contact. The Journal of cell biology. 1989;109:557–569. doi: 10.1083/jcb.109.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbelaar MA, Selten-Versteegen AM, Dunia I, Benedetti EL, Bloemendal H. Actin in mammalian lens. European journal of biochemistry / FEBS. 1979;95:543–549. doi: 10.1111/j.1432-1033.1979.tb12995.x. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Adaptors for clathrin-mediated traffic. Annual review of cell and developmental biology. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Clathrin. Annual review of biochemistry. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Harrison SC, Heuser J. Configuration of clathrin trimers: evidence from electron microscopy. Journal of ultrastructure and molecular structure research. 1986;94:199–208. doi: 10.1016/0889-1605(86)90067-4. [DOI] [PubMed] [Google Scholar]
- Kistler J, Gilbert K, Brooks HV, Jolly RD, Hopcroft DH, Bullivant S. Membrane interlocking domains in the lens. Investigative ophthalmology & visual science. 1986;27:1527–1534. [PubMed] [Google Scholar]
- Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BL, Bennett V. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. The Journal of biological chemistry. 2007;282:26552–26561. doi: 10.1074/jbc.M703158200. [DOI] [PubMed] [Google Scholar]
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Current biology : CB. 2002;12:379–382. doi: 10.1016/s0960-9822(02)00661-9. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. The Journal of biological chemistry. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- Kuszak J, Alcala J, Maisel H. The surface morphology of embryonic and adult chick lens-fiber cells. The American journal of anatomy. 1980;159:395–410. doi: 10.1002/aja.1001590406. [DOI] [PubMed] [Google Scholar]
- Kuszak JR. The ultrastructure of epithelial and fiber cells in the crystalline lens. International review of cytology. 1995;163:305–350. doi: 10.1016/s0074-7696(08)62213-5. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Sivak JG, Weerheim JA. Lens optical quality is a direct function of lens sutural architecture. Investigative ophthalmology & visual science. 1991;32:2119–2129. [PubMed] [Google Scholar]
- Kuszak JR, Zoltoski RK, Sivertson C. Fibre cell organization in crystalline lenses. Experimental eye research. 2004a;78:673–687. doi: 10.1016/j.exer.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Zoltoski RK, Tiedemann CE. Development of lens sutures. The International journal of developmental biology. 2004b;48:889–902. doi: 10.1387/ijdb.041880jk. [DOI] [PubMed] [Google Scholar]
- Kuwabara T. Microtubules in the lens. Archives of ophthalmology. 1968;79:189–195. doi: 10.1001/archopht.1968.03850040191017. [DOI] [PubMed] [Google Scholar]
- Kuwabara T. The maturation of the lens cell: a morphologic study. Experimental eye research. 1975;20:427–443. doi: 10.1016/0014-4835(75)90085-8. [DOI] [PubMed] [Google Scholar]
- Lang RA, Herman K, Reynolds AB, Hildebrand JD, Plageman TF., Jr p120-catenin-dependent junctional recruitment of Shroom3 is required for apical constriction during lens pit morphogenesis. Development. 2014;141:3177–3187. doi: 10.1242/dev.107433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Fischer RS, Fowler VM. Stabilization and remodeling of the membrane skeleton during lens fiber cell differentiation and maturation. Developmental dynamics : an official publication of the American Association of Anatomists. 2000;217:257–270. doi: 10.1002/(SICI)1097-0177(200003)217:3<257::AID-DVDY4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lee A, Morrow JS, Fowler VM. Caspase remodeling of the spectrin membrane skeleton during lens development and aging. The Journal of biological chemistry. 2001;276:20735–20742. doi: 10.1074/jbc.M009723200. [DOI] [PubMed] [Google Scholar]
- Leeson TS. Lens of the rat eye: an electron microscope and freeze-etch study. Experimental eye research. 1971;11:78–82. doi: 10.1016/s0014-4835(71)80067-2. [DOI] [PubMed] [Google Scholar]
- Leng Y, Zhang J, Badour K, Arpaia E, Freeman S, Cheung P, Siu M, Siminovitch K. Abelson-interactor-1 promotes WAVE2 membrane translocation and Abelson-mediated tyrosine phosphorylation required for WAVE2 activation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1098–1103. doi: 10.1073/pnas.0409120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard M, Zhang L, Zhai N, Cader A, Chan Y, Nowak RB, Fowler VM, Menko AS. Modulation of N-cadherin junctions and their role as epicenters of differentiation-specific actin regulation in the developing lens. Developmental biology. 2011;349:363–377. doi: 10.1016/j.ydbio.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong L, Menko AS, Grunwald GB. Differential expression of N- and B-cadherin during lens development. Investigative ophthalmology & visual science. 2000;41:3503–3510. [PubMed] [Google Scholar]
- Li L, Chang B, Cheng C, Chang D, Hawes NL, Xia CH, Gong X. Dense nuclear cataract caused by the gammaB-crystallin S11R point mutation. Investigative ophthalmology & visual science. 2008;49:304–309. doi: 10.1167/iovs.07-0942. [DOI] [PubMed] [Google Scholar]
- Lin Q, Zhou N, Zhang N, Zhu B, Hu S, Zhou Z, Qi Y. Genetic variations and polymorphisms in the ezrin gene are associated with age-related cataract. Molecular vision. 2013;19:1572–1579. [PMC free article] [PubMed] [Google Scholar]
- Linseman DA, Loucks FA. Diverse roles of Rho family GTPases in neuronal development, survival, and death. Frontiers in bioscience : a journal and virtual library. 2008;13:657–676. doi: 10.2741/2710. [DOI] [PubMed] [Google Scholar]
- Liou W, Rafferty NS. Actin filament patterns in mouse lens epithelium: a study of the effects of aging, injury, and genetics. Cell motility and the cytoskeleton. 1988;9:17–29. doi: 10.1002/cm.970090104. [DOI] [PubMed] [Google Scholar]
- Lo WK. Adherens junctions in the ocular lens of various species: ultrastructural analysis with an improved fixation. Cell and tissue research. 1988;254:31–40. doi: 10.1007/BF00220014. [DOI] [PubMed] [Google Scholar]
- Lo WK, Biswas SK, Brako L, Shiels A, Gu S, Jiang JX. Aquaporin-0 targets interlocking domains to control the integrity and transparency of the eye lens. Investigative ophthalmology & visual science. 2014;55:1202–1212. doi: 10.1167/iovs.13-13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WK, Harding CV. Square arrays and their role in ridge formation in human lens fibers. Journal of ultrastructure research. 1984;86:228–245. doi: 10.1016/s0022-5320(84)90103-5. [DOI] [PubMed] [Google Scholar]
- Lo WK, Reese TS. Multiple structural types of gap junctions in mouse lens. Journal of cell science. 1993;106(Pt 1):227–235. doi: 10.1242/jcs.106.1.227. [DOI] [PubMed] [Google Scholar]
- Lo WK, Shaw AP, Wen XJ. Actin filament bundles in cortical fiber cells of the rat lens. Experimental eye research. 1997;65:691–701. doi: 10.1006/exer.1997.0375. [DOI] [PubMed] [Google Scholar]
- Lo WK, Wen XJ, Zhou CJ. Microtubule configuration and membranous vesicle transport in elongating fiber cells of the rat lens. Experimental eye research. 2003;77:615–626. doi: 10.1016/s0014-4835(03)00176-3. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Robinson ML. Development of the ocular lens. Cambridge, UK ; New York: Cambridge University Press; 2004. [Google Scholar]
- Luck S, Choh V. Effects of a myosin light chain kinase inhibitor on the optics and accommodation of the avian crystalline lens. Molecular vision. 2011;17:2759–2764. [PMC free article] [PubMed] [Google Scholar]
- Luna EJ, Hitt AL. Cytoskeleton--plasma membrane interactions. Science. 1992;258:955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Maddala R, Chauhan BK, Walker C, Zheng Y, Robinson ML, Lang RA, Rao PV. Rac1 GTPase-deficient mouse lens exhibits defects in shape, suture formation, fiber cell migration and survival. Developmental biology. 2011a;360:30–43. doi: 10.1016/j.ydbio.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddala R, Deng PF, Costello JM, Wawrousek EF, Zigler JS, Rao VP. Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse. Laboratory investigation; a journal of technical methods and pathology. 2004;84:679–692. doi: 10.1038/labinvest.3700105. [DOI] [PubMed] [Google Scholar]
- Maddala R, Nagendran T, Lang RA, Morozov A, Rao PV. Rap1 GTPase Is required for mouse lens epithelial maintenance and morphogenesis. Developmental biology. 2015a doi: 10.1016/j.ydbio.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddala R, Skiba N, Vasantha Rao P. Lens fiber cell elongation and differentiation is associated with a robust increase in myosin light chain phosphorylation in the developing mouse. Differentiation; research in biological diversity. 2007;75:713–725. doi: 10.1111/j.1432-0436.2007.00173.x. [DOI] [PubMed] [Google Scholar]
- Maddala R, Skiba NP, Lalane R, 3rd, Sherman DL, Brophy PJ, Rao PV. Periaxin is required for hexagonal geometry and membrane organization of mature lens fibers. Developmental biology. 2011b;357:179–190. doi: 10.1016/j.ydbio.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddala R, Walters M, Brophy PJ, Bennett V, Rao PV. Ankyrin-B directs membrane tethering of Periaxin and is required for maintenance of lens fiber cell hexagonal shape and mechanics. American journal of physiology. Cell physiology. 2015b doi: 10.1152/ajpcell.00111.2015. ajpcell 00111 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchanda N, Lyubimova A, Ho HY, James MF, Gusella JF, Ramesh N, Snapper SB, Ramesh V. The NF2 tumor suppressor Merlin and the ERM proteins interact with N-WASP and regulate its actin polymerization function. The Journal of biological chemistry. 2005;280:12517–12522. doi: 10.1074/jbc.C400583200. [DOI] [PubMed] [Google Scholar]
- Mann I. The development of the human eye. 3d. New York: Grune & Stratton; 1964. [Google Scholar]
- Matsudaira P. Actin crosslinking proteins at the leading edge. Seminars in cell biology. 1994;5:165–174. doi: 10.1006/scel.1994.1021. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Li X, Bennett V. Adducin: structure, function and regulation. Cellular and molecular life sciences : CMLS. 2000;57:884–895. doi: 10.1007/PL00000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy JW. Cytoplasmic processes interconnect lens placode and optic vesicle during eye morphogenesis. Experimental eye research. 1980;31:527–534. doi: 10.1016/s0014-4835(80)80011-x. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Miki H, Takenawa T. Regulation of actin dynamics by WASP family proteins. Journal of biochemistry. 2003;134:309–313. doi: 10.1093/jb/mvg146. [DOI] [PubMed] [Google Scholar]
- Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- More MI, Kirsch FP, Rathjen FG. Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers leading to cataract formation. The Journal of cell biology. 2001;154:187–196. doi: 10.1083/jcb.200104038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nature cell biology. 2000;2:376–378. doi: 10.1038/35014075. [DOI] [PubMed] [Google Scholar]
- Mousa GY, Trevithick JR. Differentiation of rat lens epithelial cells in tissue culture. II. Effects of cytochalasins B and D on actin organization and differentiation. Developmental biology. 1977;60:14–25. doi: 10.1016/0012-1606(77)90107-5. [DOI] [PubMed] [Google Scholar]
- Moyer JD, Nowak RB, Kim NE, Larkin SK, Peters LL, Hartwig J, Kuypers FA, Fowler VM. Tropomodulin 1-null mice have a mild spherocytic elliptocytosis with appearance of tropomodulin 3 in red blood cells and disruption of the membrane skeleton. Blood. 2010;116:2590–2599. doi: 10.1182/blood-2010-02-268458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli M, Qaisi D, Herman K, Plageman TF., Jr Lens placode planar cell polarity is dependent on Cdc42-mediated junctional contraction inhibition. Developmental biology. 2016 doi: 10.1016/j.ydbio.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Larsson M, Robinson RC, Burtnick LD. Gelsolin: the tail of a molecular gymnast. Cytoskeleton. 2013;70:360–384. doi: 10.1002/cm.21117. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. The Journal of cell biology. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan P, Chatterton P, Ikeda A, Ikeda S, Corey DP, Ervasti JM, Perrin BJ. Length regulation of mechanosensitive stereocilia depends on very slow actin dynamics and filament-severing proteins. Nature communications. 2015;6:6855. doi: 10.1038/ncomms7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MM, Rivera C, Griep AE. Localization of PDZ domain containing proteins Discs Large-1 and Scribble in the mouse eye. Molecular vision. 2005;11:1183–1199. [PubMed] [Google Scholar]
- Nishida E, Maekawa S, Sakai H. Cofilin, a protein in porcine brain that binds to actin filaments and inhibits their interactions with myosin and tropomyosin. Biochemistry. 1984;23:5307–5313. doi: 10.1021/bi00317a032. [DOI] [PubMed] [Google Scholar]
- Nobes C, Hall A. Regulation and function of the Rho subfamily of small GTPases. Current opinion in genetics & development. 1994;4:77–81. doi: 10.1016/0959-437x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. The Journal of cell biology. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen BJ, Tomasevic N, Russell A, Pierce DW, Jia Z, McCormick CD, Hartman J, Sakowicz R, Pollard TD. Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature. 2009;460:1031–1034. doi: 10.1038/nature08231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak RB, Fischer RS, Zoltoski RK, Kuszak JR, Fowler VM. Tropomodulin1 is required for membrane skeleton organization and hexagonal geometry of fiber cells in the mouse lens. The Journal of cell biology. 2009;186:915–928. doi: 10.1083/jcb.200905065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak RB, Fowler VM. Tropomodulin 1 constrains fiber cell geometry during elongation and maturation in the lens cortex. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2012;60:414–427. doi: 10.1369/0022155412440881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M, Kudo H, Sugama N, Asami Y, Takehana M. The function of filensin and phakinin in lens transparency. Molecular vision. 2008;14:815–822. [PMC free article] [PubMed] [Google Scholar]
- Ono S. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. International review of cytology. 2007;258:1–82. doi: 10.1016/S0074-7696(07)58001-0. [DOI] [PubMed] [Google Scholar]
- Ono S. Dynamic regulation of sarcomeric actin filaments in striated muscle. Cytoskeleton. 2010;67:677–692. doi: 10.1002/cm.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Weinberg RA. A putative effector of Ral has homology to Rho/Rac GTPase activating proteins. Oncogene. 1995;11:2349–2355. [PubMed] [Google Scholar]
- Pearse BM, Smith CJ, Owen DJ. Clathrin coat construction in endocytosis. Current opinion in structural biology. 2000;10:220–228. doi: 10.1016/s0959-440x(00)00071-3. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens cell elongation in vitro and microtubules. Annals of the New York Academy of Sciences. 1975;253:333–347. doi: 10.1111/j.1749-6632.1975.tb19211.x. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation; research in biological diversity. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Pieters T, van Hengel J, van Roy F. Functions of p120ctn in development and disease. Frontiers in bioscience. 2012;17:760–783. doi: 10.2741/3956. [DOI] [PubMed] [Google Scholar]
- Plageman TF, Jr, Chauhan BK, Yang C, Jaudon F, Shang X, Zheng Y, Lou M, Debant A, Hildebrand JD, Lang RA. A Trio-RhoA-Shroom3 pathway is required for apical constriction and epithelial invagination. Development. 2011;138:5177–5188. doi: 10.1242/dev.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plageman TF, Jr, Chung MI, Lou M, Smith AN, Hildebrand JD, Wallingford JB, Lang RA. Pax6-dependent Shroom3 expression regulates apical constriction during lens placode invagination. Development. 2010;137:405–415. doi: 10.1242/dev.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocha SM, Montell DJ. Cellular and molecular mechanisms of single and collective cell migrations in Drosophila: themes and variations. Annual review of genetics. 2014;48:295–318. doi: 10.1146/annurev-genet-120213-092218. [DOI] [PubMed] [Google Scholar]
- Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annual review of biophysics and biomolecular structure. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annual review of biophysics and biomolecular structure. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA. Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Developmental biology. 2009;326:403–417. doi: 10.1016/j.ydbio.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, Boone C. Role of formins in actin assembly: nucleation and barbed-end association. Science. 2002;297:612–615. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- Quintin S, Gally C, Labouesse M. Epithelial morphogenesis in embryos: asymmetries, motors and brakes. Trends in genetics : TIG. 2008;24:221–230. doi: 10.1016/j.tig.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Rafferty NS. Lens Morphology. New York: Dekker; 1985. [Google Scholar]
- Rafferty NS, Scholz DL. Polygonal arrays of microfilaments in epithelial cells of the intact lens. Current eye research. 1984;3:1141–1149. doi: 10.3109/02713688409000814. [DOI] [PubMed] [Google Scholar]
- Rafferty NS, Scholz DL. Actin in polygonal arrays of microfilaments and sequestered actin bundles (SABs) in lens epithelial cells of rabbits and mice. Current eye research. 1985;4:713–718. doi: 10.3109/02713688509017667. [DOI] [PubMed] [Google Scholar]
- Rafferty NS, Scholz DL. Comparative study of actin filament patterns in lens epithelial cells. Are these determined by the mechanisms of lens accommodation? Current eye research. 1989;8:569–579. doi: 10.3109/02713688908995756. [DOI] [PubMed] [Google Scholar]
- Rafferty NS, Scholz DL, Goldberg M, Lewyckyj M. Immunocytochemical evidence for an actin-myosin system in lens epithelial cells. Experimental eye research. 1990;51:591–600. doi: 10.1016/0014-4835(90)90090-h. [DOI] [PubMed] [Google Scholar]
- Ramaekers FC, Boomkens TR, Bloemendal H. Cytoskeletal and contractile structures in bovine lens cell differentiation. Experimental cell research. 1981;135:454–461. doi: 10.1016/0014-4827(81)90190-7. [DOI] [PubMed] [Google Scholar]
- Rao PV, Ho T, Skiba NP, Maddala R. Characterization of lens fiber cell triton insoluble fraction reveals ERM (ezrin, radixin, moesin) proteins as major cytoskeletal-associated proteins. Biochemical and biophysical research communications. 2008;368:508–514. doi: 10.1016/j.bbrc.2008.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V, Wawrousek E, Tamm ER, Zigler S., Jr Rho GTPase inactivation impairs lens growth and integrity. Laboratory investigation; a journal of technical methods and pathology. 2002;82:231–239. doi: 10.1038/labinvest.3780415. [DOI] [PubMed] [Google Scholar]
- Ren G, Crampton MS, Yap AS. Cortactin: Coordinating adhesion and the actin cytoskeleton at cellular protrusions. Cell motility and the cytoskeleton. 2009;66:865–873. doi: 10.1002/cm.20380. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Riedl J, Flynn KC, Raducanu A, Gartner F, Beck G, Bosl M, Bradke F, Massberg S, Aszodi A, Sixt M, Wedlich-Soldner R. Lifeact mice for studying F-actin dynamics. Nature methods. 2010;7:168–169. doi: 10.1038/nmeth0310-168. [DOI] [PubMed] [Google Scholar]
- Rivera C, Yamben IF, Shatadal S, Waldof M, Robinson ML, Griep AE. Cell-autonomous requirements for Dlg-1 for lens epithelial cell structure and fiber cell morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:2292–2308. doi: 10.1002/dvdy.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SA, Neidt EM, Cui J, Feiger Z, Skau CT, Gardel ML, Kozmin SA, Kovar DR. Identification and characterization of a small molecule inhibitor of formin-mediated actin assembly. Chemistry & biology. 2009;16:1158–1168. doi: 10.1016/j.chembiol.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffers-Agarwal J, Xanthos JB, Kragtorp KA, Miller JR. Enabled (Xena) regulates neural plate morphogenesis, apical constriction, and cellular adhesion required for neural tube closure in Xenopus. Developmental biology. 2008;314:393–403. doi: 10.1016/j.ydbio.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JR, Echarri A, Li R, Pendergast AM. Regulation of cell-cell adhesion by Abi/Diaphanous complexes. Molecular and cellular biology. 2009;29:1735–1748. doi: 10.1128/MCB.01483-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot I, Rodal AA, Moseley J, Goode BL, Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nature cell biology. 2002;4:626–631. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- Sahu M, Sharma R, Yadav S, Wakamiya M, Chaudhary P, Awasthi S, Awasthi YC. Lens specific RLIP76 transgenic mice show a phenotype similar to microphthalmia. Experimental eye research. 2014;118:125–134. doi: 10.1016/j.exer.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Prescott AR, Carter JM, Hutcheson AM, Quinlan RA, Richards J, FitzGerald PG. Vimentin and CP49/filensin form distinct networks in the lens which are independently modulated during lens fibre cell differentiation. Journal of cell science. 1995;108(Pt 4):1397–1406. doi: 10.1242/jcs.108.4.1397. [DOI] [PubMed] [Google Scholar]
- Saposnik B, Binard S, Fenneteau O, Nurden A, Nurden P, Hurtaud-Roux MF, Schlegel N, French MYHn. Mutation spectrum and genotype-phenotype correlations in a large French cohort of MYH9-Related Disorders. Molecular genetics & genomic medicine. 2014;2:297–312. doi: 10.1002/mgg3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Current opinion in cell biology. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Scholz DL, Rafferty NS. Immunogold-EM localization of actin and vimentin filaments in relation to polygonal arrays in lens epithelium in situ. Current eye research. 1988;7:705–719. doi: 10.3109/02713688809033200. [DOI] [PubMed] [Google Scholar]
- Shang X, Marchioni F, Evelyn CR, Sipes N, Zhou X, Seibel W, Wortman M, Zheng Y. Small-molecule inhibitors targeting G-protein-coupled Rho guanine nucleotide exchange factors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3155–3160. doi: 10.1073/pnas.1212324110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X, Marchioni F, Sipes N, Evelyn CR, Jerabek-Willemsen M, Duhr S, Seibel W, Wortman M, Zheng Y. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chemistry & biology. 2012;19:699–710. doi: 10.1016/j.chembiol.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoblom B, Salmazo A, Djinovic-Carugo K. Alpha-actinin structure and regulation. Cellular and molecular life sciences : CMLS. 2008;65:2688–2701. doi: 10.1007/s00018-008-8080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Pearse BM. Clathrin: anatomy of a coat protein. Trends in cell biology. 1999;9:335–338. doi: 10.1016/s0962-8924(99)01631-1. [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins--novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell motility and the cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- Sternberg SH, Doudna JA. Expanding the Biologist's Toolkit with CRISPR-Cas9. Molecular cell. 2015;58:568–574. doi: 10.1016/j.molcel.2015.02.032. [DOI] [PubMed] [Google Scholar]
- Stradal TE, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends in cell biology. 2004;14:303–311. doi: 10.1016/j.tcb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Straub BK, Boda J, Kuhn C, Schnoelzer M, Korf U, Kempf T, Spring H, Hatzfeld M, Franke WW. A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. Journal of cell science. 2003;116:4985–4995. doi: 10.1242/jcs.00815. [DOI] [PubMed] [Google Scholar]
- Stuart JR, Gonzalez FH, Kawai H, Yuan ZM. c-Abl interacts with the WAVE2 signaling complex to induce membrane ruffling and cell spreading. The Journal of biological chemistry. 2006;281:31290–31297. doi: 10.1074/jbc.M602389200. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Akimoto K, Robinson ML, Ohno S, Quinlan RA. A cell polarity protein aPKClambda is required for eye lens formation and growth. Developmental biology. 2009;336:246–256. doi: 10.1016/j.ydbio.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung LA, Lo WK. Immunodetection of membrane skeletal protein 4.2 in bovine and chicken eye lenses and erythrocytes. Current eye research. 1997;16:1127–1133. doi: 10.1076/ceyr.16.11.1127.5103. [DOI] [PubMed] [Google Scholar]
- Sussman MA, McAvoy JW, Rudisill M, Swanson B, Lyons GE, Kedes L, Blanks J. Lens tropomodulin: developmental expression during differentiation. Experimental eye research. 1996;63:223–232. doi: 10.1006/exer.1996.0111. [DOI] [PubMed] [Google Scholar]
- Taylor VL, al-Ghoul KJ, Lane CW, Davis VA, Kuszak JR, Costello MJ. Morphology of the normal human lens. Investigative ophthalmology & visual science. 1996;37:1396–1410. [PubMed] [Google Scholar]
- Tseng Y, Kole TP, Lee JS, Fedorov E, Almo SC, Schafer BW, Wirtz D. How actin crosslinking and bundling proteins cooperate to generate an enhanced cell mechanical response. Biochemical and biophysical research communications. 2005;334:183–192. doi: 10.1016/j.bbrc.2005.05.205. [DOI] [PubMed] [Google Scholar]
- Uruno T, Liu J, Zhang P, Fan Y, Egile C, Li R, Mueller SC, Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nature cell biology. 2001;3:259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Villalonga P, Ridley AJ. Rho GTPases and cell cycle control. Growth factors. 2006;24:159–164. doi: 10.1080/08977190600560651. [DOI] [PubMed] [Google Scholar]
- Wang Z, Schey KL. Aquaporin-0 interacts with the FERM domain of ezrin/radixin/moesin proteins in the ocular lens. Investigative ophthalmology & visual science. 2011;52:5079–5087. doi: 10.1167/iovs.10-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Current biology : CB. 2001;11:370–374. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- Weber A, Pennise CR, Babcock GG, Fowler VM. Tropomodulin caps the pointed ends of actin filaments. The Journal of cell biology. 1994;127:1627–1635. doi: 10.1083/jcb.127.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GF, Menko AS. Actin filament organization regulates the induction of lens cell differentiation and survival. Developmental biology. 2006;295:714–729. doi: 10.1016/j.ydbio.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Weitzer G, Wiche G. Plectin from bovine lenses. Chemical properties, structural analysis and initial identification of interaction partners. European journal of biochemistry / FEBS. 1987;169:41–52. doi: 10.1111/j.1432-1033.1987.tb13578.x. [DOI] [PubMed] [Google Scholar]
- Wiche G, Osmanagic-Myers S, Castanon MJ. Networking and anchoring through plectin: a key to IF functionality and mechanotransduction. Current opinion in cell biology. 2015;32:21–29. doi: 10.1016/j.ceb.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Wiche G, Winter L. Plectin isoforms as organizers of intermediate filament cytoarchitecture. Bioarchitecture. 2011;1:14–20. doi: 10.4161/bioa.1.1.14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willekens B, Vrensen G. The three-dimensional organization of lens fibers in the rabbit. A scanning electron microscopic reinvestigation. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. Albrecht von Graefe's archive for clinical and experimental ophthalmology. 1981;216:275–289. doi: 10.1007/BF00455035. [DOI] [PubMed] [Google Scholar]
- Willekens B, Vrensen G. The three-dimensional organization of lens fibers in the rhesus monkey. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1982;219:112–120. doi: 10.1007/BF02152295. [DOI] [PubMed] [Google Scholar]
- Willekens B, Vrensen G. Lens fiber organization in four avian species: a scanning electron microscopic study. Tissue & cell. 1985;17:359–377. doi: 10.1016/0040-8166(85)90055-2. [DOI] [PubMed] [Google Scholar]
- Won GJ, Fudge DS, Choh V. The effects of actomyosin disruptors on the mechanical integrity of the avian crystalline lens. Molecular vision. 2015;21:98–109. [PMC free article] [PubMed] [Google Scholar]
- Woo MK, Fowler VM. Identification and characterization of tropomodulin and tropomyosin in the adult rat lens. Journal of cell science. 1994;107(Pt 5):1359–1367. doi: 10.1242/jcs.107.5.1359. [DOI] [PubMed] [Google Scholar]
- Woo MK, Lee A, Fischer RS, Moyer J, Fowler VM. The lens membrane skeleton contains structures preferentially enriched in spectrin-actin or tropomodulin-actin complexes. Cell motility and the cytoskeleton. 2000;46:257–268. doi: 10.1002/1097-0169(200008)46:4<257::AID-CM3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Woods A, Wang G, Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. The Journal of biological chemistry. 2005;280:11626–11634. doi: 10.1074/jbc.M409158200. [DOI] [PubMed] [Google Scholar]
- Woychik RP, Generoso WM, Russell LB, Cain KT, Cacheiro NL, Bultman SJ, Selby PB, Dickinson ME, Hogan BL, Rutledge JC. Molecular and genetic characterization of a radiation-induced structural rearrangement in mouse chromosome 2 causing mutations at the limb deformity and agouti loci. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:2588–2592. doi: 10.1073/pnas.87.7.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Wu W, Tholozan FM, Saunter CD, Girkin JM, Quinlan RA. A dimensionless ordered pull-through model of the mammalian lens epithelium evidences scaling across species and explains the age-dependent changes in cell density in the human lens. Journal of the Royal Society, Interface / the Royal Society. 2015;12 doi: 10.1098/rsif.2015.0391. 20150391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Overbeek PA, Reneker LW. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Experimental eye research. 2002;74:753–760. doi: 10.1006/exer.2002.1175. [DOI] [PubMed] [Google Scholar]
- Yamashiro S, Gokhin DS, Kimura S, Nowak RB, Fowler VM. Tropomodulins: pointed-end capping proteins that regulate actin filament architecture in diverse cell types. Cytoskeleton. 2012;69:337–370. doi: 10.1002/cm.21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S, Scholz DL, Liou W, Rafferty NS. Polygonal arrays of actin filaments in human lens epithelial cells. An aging study. Investigative ophthalmology & visual science. 1986;27:1535–1540. [PubMed] [Google Scholar]
- Yin HL, Stossel TP. Purification and structural properties of gelsolin, a Ca2+activated regulatory protein of macrophages. The Journal of biological chemistry. 1980;255:9490–9493. [PubMed] [Google Scholar]
- Zampighi GA, Eskandari S, Kreman M. Epithelial organization of the mammalian lens. Experimental eye research. 2000;71:415–435. doi: 10.1006/exer.2000.0895. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Conti MA, Malide D, Dong F, Wang A, Shmist YA, Liu C, Zerfas P, Daniels MP, Chan CC, Kozin E, Kachar B, Kelley MJ, Kopp JB, Adelstein RS. Mouse models of MYH9-related disease: mutations in nonmuscle myosin II-A. Blood. 2012;119:238–250. doi: 10.1182/blood-2011-06-358853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CJ, Lo WK. Association of clathrin, AP-2 adaptor and actin cytoskeleton with developing interlocking membrane domains of lens fibre cells. Experimental eye research. 2003;77:423–432. doi: 10.1016/s0014-4835(03)00171-4. [DOI] [PubMed] [Google Scholar]