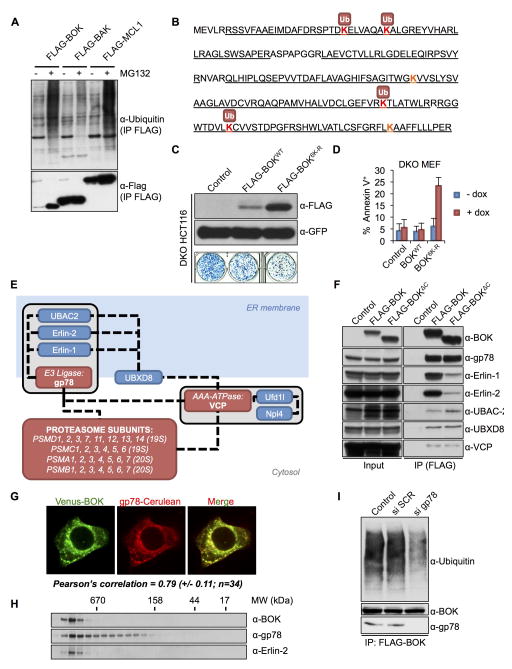
Figure 3. BOK stability is regulated by the ERAD pathway.
A. Ubiquitylation profile of Flag-tagged BOK, BAK, or MCL-1 immunoprecipitated from transiently transfected HCT116 cells treated with 40 μM Q-VD-POh ±10 μM MG132. B. Ubiquitylation sites in murine BOK identified by MS (peptide coverage underlined). C. Clonogenic survival of bax−/−bak−/− HCT116 cells transiently transfected with an empty pMX-IRES-GFP or expressing FLAG-BOKWT or FLAG-BOK6K-R and sorted for equivalent GFP intensity (lower panel). Upper panel: immunoblot of FLAG and GFP. D. Flow cytometry quantification of Annexin V–stained bax−/−bak−/− MEFs expressing Venus2ABOKWT or Venus2ABOK6K-R and treated with dox for 24 h (mean ± SD of 3 independent experiments) E. BOK interacting network as identified by tandem affinity purification and MS analysis. F. Immunoprecipitation of Flag-BOKFL and Flag-BOKΔC from lysates of bax−/−bak−/− MEFs treated with dox, MG132 (10 μM), and Q-VD-OPh (40 μM). G. Confocal microscopy images of HeLa cells cotransfected with Venus-BOK and gp78-Cerulean in presence of MG132 (10 μM) and Q-VD-OPh. Pearson correlation coefficient ± SD is indicated. H. Size exclusion chromatography of cell lysates from MEFs expressing Venus2ABOK and treated with dox, MG132 (10 μM), and Q-VD-OPh (40 μM). I. Ubiquitylation profile of Flag-tagged BOK immunoprecipitated from bax−/−bak−/− MEFs treated with scrambled (SCR) or gp78 targeting siRNA and dox, MG132 (10 μM), and Q-VD-OPh (40 μM) (see also Figure S3).