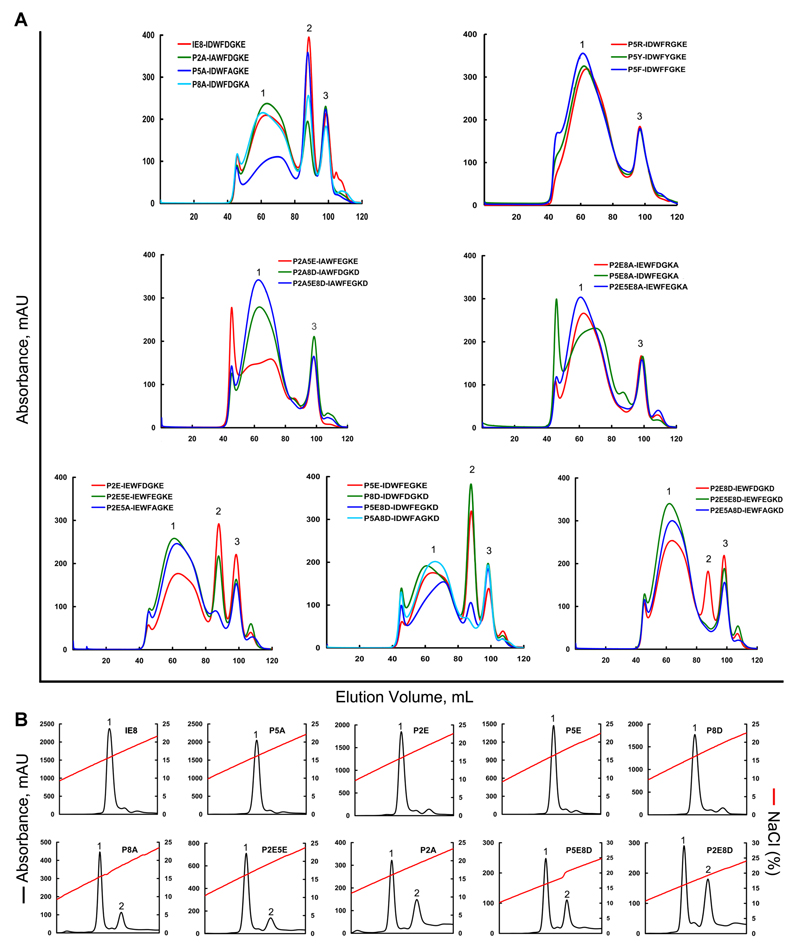
Figure 6. Refolding of BF2*0401 and β2m with IE8 and its mutants.
Peptide-induced assembly and stabilization assay of BF2*0401 molecules by in vitro refolding. (A) Gel filtration chromatograms of the refolded products. Peak 1, peak 2 and peak 3 represent the aggregated heavy chain, the correctly refolded BF2*0401 complex (45 kDa) and the extra β2m, respectively. The refolding efficiencies are represented by the relevant concentration ratio and height of the peak 2 for IE8 and each mutant, the more the better. Otherwise, if little or none peak 2 will be observed, the peptide can not be considered to stabilize the complex, thereof treated a non-presenting peptide. The IE8 mutants were listed in Table II. (B) Results of further stabilization assays of the refolded complexes by anion exchange. Under the anion-exchange conditions, complexes of IE8, P5A, P2E, P5E and P8D can be eluted normally at NaCl concentration of 15%-17% (peak 1). With the peptides of P8A, P2E5E, P2A, P5E8D and P2E8D, the refolded complex proteins can be partially dissociated at NaCl concentration of 18%-20% (peak 2), implying less stable.